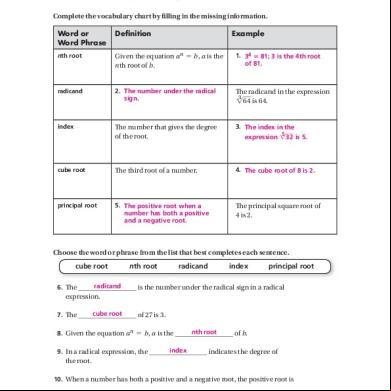
Lab 19c Ans Sheet 9663o
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 2z6p3t
Overview 5o1f4z
& View Lab 19c Ans Sheet as PDF for free.
More details 6z3438
- Words: 390
- Pages: 4
Chemistry 12 Lab Manual
Lab # :19c________________________ Title: Determination of a Solubility Product Constant Name: _______________ Date: ________________ Mrs. Taylor
Objectives: State all the objectives listed in the lab.
Data Table: Test Tube #
A
B
C
D
E
F
Vol 0.010M Pb(NO3)2 Volume of H2O added (ml) Volume of 0.020 M KI (ml) Volume of H2O added (ml) Precipitate or no Precipitate Temperature at which Precitpate dissolves
Questions and Calculations: 1.
For each of test tubes A to F calculate the [Pb2+] in the final mixed solution. In using the appropriate dilution factor, that the final volume in each case is the sum of the volumes of both the lead nitrate and the potassium iodide, i.e., 20 mL
Adapted from Heath Chemistry 18A
Page- 5 -
©Lockwood
Chemistry 12 Lab Manual
2.
Repeat for [I-] in the final mixed solution in test tubes A to F.
3.
Calculate the value of the trial ion product for each of test tubes A to F (given by [Pb2+][I-]2.).
4.
State the range of values in which your experimental Ksp must lie. (This will be between the trial ion product of the last test tube giving a precipitate and the trial ion product of the first test tube not giving a precipitate).
5.
From the results of the temperature at which a precipitate dissolved, make up a table showing the temperature, the Ksp' and the solubility in each case. (Since in each situation the [I-] was twice the [Pb2+], the solubility of PbI2 is given simply by the [Pb2+] alone.)
6.
What is the trend in the solubility as the temperature is increased?
7.
Plot a graph of solubility (moles per litre) against temperature.
Follow up Questions: 1. If you were given a saturated solution of lead iodide and asked to determine the KSP of PbI2 from it, how would you proceed? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________
Adapted from Heath Chemistry 18A
Page- 6 -
©Lockwood
Chemistry 12 Lab Manual
2. Would lead iodide be more or less soluble in a solition of 0.10M KI than it will be in pure water? Explain your answer? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________
Conclusion: State the results of Objective 3. ________________________________________________________________________ ________________________________________________________________________ ______________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________
Adapted from Heath Chemistry 18A
Page- 7 -
©Lockwood
Chemistry 12 Lab Manual
Adapted from Heath Chemistry 18A
Page- 8 -
©Lockwood
Lab # :19c________________________ Title: Determination of a Solubility Product Constant Name: _______________ Date: ________________ Mrs. Taylor
Objectives: State all the objectives listed in the lab.
Data Table: Test Tube #
A
B
C
D
E
F
Vol 0.010M Pb(NO3)2 Volume of H2O added (ml) Volume of 0.020 M KI (ml) Volume of H2O added (ml) Precipitate or no Precipitate Temperature at which Precitpate dissolves
Questions and Calculations: 1.
For each of test tubes A to F calculate the [Pb2+] in the final mixed solution. In using the appropriate dilution factor, that the final volume in each case is the sum of the volumes of both the lead nitrate and the potassium iodide, i.e., 20 mL
Adapted from Heath Chemistry 18A
Page- 5 -
©Lockwood
Chemistry 12 Lab Manual
2.
Repeat for [I-] in the final mixed solution in test tubes A to F.
3.
Calculate the value of the trial ion product for each of test tubes A to F (given by [Pb2+][I-]2.).
4.
State the range of values in which your experimental Ksp must lie. (This will be between the trial ion product of the last test tube giving a precipitate and the trial ion product of the first test tube not giving a precipitate).
5.
From the results of the temperature at which a precipitate dissolved, make up a table showing the temperature, the Ksp' and the solubility in each case. (Since in each situation the [I-] was twice the [Pb2+], the solubility of PbI2 is given simply by the [Pb2+] alone.)
6.
What is the trend in the solubility as the temperature is increased?
7.
Plot a graph of solubility (moles per litre) against temperature.
Follow up Questions: 1. If you were given a saturated solution of lead iodide and asked to determine the KSP of PbI2 from it, how would you proceed? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________
Adapted from Heath Chemistry 18A
Page- 6 -
©Lockwood
Chemistry 12 Lab Manual
2. Would lead iodide be more or less soluble in a solition of 0.10M KI than it will be in pure water? Explain your answer? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________
Conclusion: State the results of Objective 3. ________________________________________________________________________ ________________________________________________________________________ ______________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________
Adapted from Heath Chemistry 18A
Page- 7 -
©Lockwood
Chemistry 12 Lab Manual
Adapted from Heath Chemistry 18A
Page- 8 -
©Lockwood