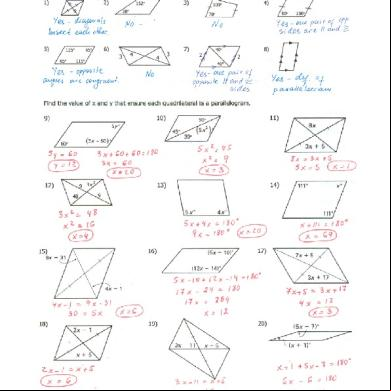
Thermochemistry Practice Sheet Answer Key a6z1j
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 2z6p3t
Overview 5o1f4z
& View Thermochemistry Practice Sheet Answer Key as PDF for free.
More details 6z3438
- Words: 1,064
- Pages: 8
CEM 111 Thermochemistry and Calorimetry Practice Sheet 1) Hydrogen peroxide decomposes according to the following thermochemical reaction: H2O2(l) --> H2O(l) + 1/2 O2(g) H = -98.2 kJ Calculate the change in enthalpy, H, when 1.00 g of hydrogen peroxide decomposes.
-2.89 kJ
2) Find H for the reaction of sulfur dioxide with oxygen to form sulfur trioxide given the following heats of formation: SO2 Hf = -296.8 kJ/mol SO3 Hf = -395.7 kJ/mol
-197.8 kJ
3) From the following data, calculate the enthalpy of the reaction CH4(g) + 2 O2(g) -> CO2(g) + 2H2O(g). CH4(g) + 2O2(g) -> CO2(g) + 2H2O(l)
Ho = -890 kJ/mol Ho = +44 kJ/mol
H2O(l) -> H2O(g)
-802 kJ/mol
4) From the following enthalpies of reactions, calculate the heat of combustion of methane into carbon dioxide and gaseous H2O. NOTE use balanced equations. Not all of these reaction steps will be necessary. 2O(g) -> O2(g)
Ho = -249 kJ/mol
H2O(l) -> H2O(g)
Ho = +44 kJ/mol at 298K
2H(g) + O(g) -> H2O(g)
Ho = -803 kJ/mol
C(graphite) + 2O(g) -> CO2(g)
Ho = -643 kJ/mol
C(graphite) + O2(g) -> CO2(g)
Ho = -394 kJ/mol
C(graphite) + 2H2(g) -> CH4(g)
Ho = -75 kJ/mol
2H(g) -> H2(g)
Ho = -436 kJ/mol
-804 kJ/mol
5) The standard enthalpies of formation of SO2 and SO3 are -297kJ/mol and -396kJ/mol respectively. What is the standard enthalpy of the following reaction? SO3(g) -> SO2(g) + ½O2(g)
+99 kJ/mol
6) Use the bond energies given below to estimate the change in enthalpy, H, for the following reaction: CH2CH2 + Cl2 -> ClH2C2ClH2 Bond C-C C=C C - Cl C–H Cl – Cl
Bond Energies (kJ/mol) 347 612 341 414 243
-174 kJ/mol
7) Hydrogen cyanide is produced from methane and ammonia according to the following equation: CH4(g) + NH3(g) –> HCN(g) +3H2(g) Find the enthalpy change of this reaction given the following reaction steps… H2(g) + 2C(graphite) + N2(g) –> 2HCN(g) Ho = +270. kJ N2(g) + 3H2(g) –> 2NH3(g)
Ho = -92 kJ
C(graphite) + 2H2(g) –> CH4(g)
Ho = -75 kJ
+256 kJ
8) Calculate the enthalpy change of the this reaction: 2H3BO3(aq) -> B2O3(s) + 3H2O(l), given the following reaction steps… H3BO3(aq) -> HBO2(aq) + H2O(l)
Ho = -0.02kJ
H2B4O7(s) + H2O(l) -> 4HBO2(aq)
Ho = -11.3kJ
H2B4O7(s) -> 2B2O3(s) + H2O(l)
Ho = +17.5kJ
+14.3kJ
9) The specific heat of water is 4.184 J/gC. How much heat is required to change the temperature of 42.0 g water from 22.0 C to 48.5 C?
4660 J -or- 4.66 kJ
10) In an experiment, 74.8 J of heat is required to raise the temperature of 18.69 g of silver from 10.0C to 28.0 C. What is the specific heat of silver?
0.222 J/gC
11) How much heat energy in kilojoules is required to heat all the aluminum in a roll of aluminum foil (500. grams) from room temperature, 25C to the temperature of a hot oven, 225 C? The specific heat of aluminum is 0.901 J/gC.
90.1 kJ
12) A 192g piece of copper is heated to 100.0C in a boiling water bath and then dropped into a beaker containing 750. g of water at 4.0C. If the specific heat of copper is 0.385 J/gC and the specific heat of water is 4.184 J/gC, what is the final temperature of the copper and water after they come to thermal equilibrium?
6.21C
13) Molar heat capacity is the amount of heat required to change the temperature of 1 mole of a substance by 1 C (or by 1 kelvin). Calculate the amount of heat required to change the temperature of 185.0 g of zinc from 22.5 C to 58.0 C if the molar heat capacity of zinc is 25.37 J/molC.
2,550 J -or- 2.55 kJ
14) A 0.300g sample of carbon is burned in excess oxygen in a bomb calorimeter according to the following equation: C(s) + O2(g) CO2(g) The temperature of the calorimeter increases from 25.00 C to 27.38 C. Calculate the amount of energy released during the reaction. The calorimeter has a heat capacity of 893 J/C.
-2130 J -or- -2.13 kJ
15) Calculate the enthalpy of reaction for the neutralization reaction below, given the data that follows: CsOH(aq) + HCl(aq) CsCl(aq) + H2O(l) When 100.0 mL of 0.200 M CsOH were mixed with 50.0 mL of 0.400 M HCl in a coffee-cup calorimeter, the temperature of the solutions changed from 22.500C before mixing to 24.260 C after the acidbase reaction. The densities of the solutions are 1.000 g/mL. The specific heat of the solutions are 4.150 J/gC and the heat capacity of the calorimeter is 10.30 J/C.
-1114J -or- -1.114 kJ
16) Calculate Hrxn for the combustion of ethanol according to the equation below, given the information that follows: C2H5OH(l) + 3 O2(g) 2 CO2(g) + 3 H2O(l) When a 2.84 g sample of ethanol was burned in an excess of oxygen in a bomb calorimeter, which contains 815 g of water, the temperature of the calorimeter increased from 25.00 C to 44.40 C. The specific heat of water is 4.184 J/gC and the heat capacity of the calorimeter is 923 J/C (not including the water).
-8.41 x 104 J -or- -84.1kJ
17) Calculate Hsolution when sodium nitrate dissolves in water from the following data: NaNO3(s) Na+(aq) + NO3–(aq) When 15.3 grams of NaNO3 were dissolved in 253g of water in a calorimeter, the temperature fell from 25.00 C to 21.56 C. The specific heat of water is 4.184 J/gC and the heat capacity of the calorimeter (without the water) is 12.4 J/C.
+3680 J -or- +3.68 kJ
18) Calculate the enthalpy change that occurs when 36.9 g H2S are burned according to the following equation: 2H2S(g) + 3 O2(g) 2SO2(g) + 2H2O(g)
H = -1037 kJ
-561 kJ
19) Calculate the enthalpy change that occurs when 22.4 g CaCO3 decompose according to the following equation: CaCO3(s) CaO(s) + CO2(g)
H = +178.3 kJ
+39.9 kJ
20) Calculate how many grams of natural gas (CH4) must be burned to produce 50,000. kJ of heat according to the following equation: CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g)
H = -803 kJ
999g CH4
21) What is U in Joules of a system that releases 675 J of thermal energy to its surroundings and has 2,220. Joules of work done on it?
+1545 J
-2.89 kJ
2) Find H for the reaction of sulfur dioxide with oxygen to form sulfur trioxide given the following heats of formation: SO2 Hf = -296.8 kJ/mol SO3 Hf = -395.7 kJ/mol
-197.8 kJ
3) From the following data, calculate the enthalpy of the reaction CH4(g) + 2 O2(g) -> CO2(g) + 2H2O(g). CH4(g) + 2O2(g) -> CO2(g) + 2H2O(l)
Ho = -890 kJ/mol Ho = +44 kJ/mol
H2O(l) -> H2O(g)
-802 kJ/mol
4) From the following enthalpies of reactions, calculate the heat of combustion of methane into carbon dioxide and gaseous H2O. NOTE use balanced equations. Not all of these reaction steps will be necessary. 2O(g) -> O2(g)
Ho = -249 kJ/mol
H2O(l) -> H2O(g)
Ho = +44 kJ/mol at 298K
2H(g) + O(g) -> H2O(g)
Ho = -803 kJ/mol
C(graphite) + 2O(g) -> CO2(g)
Ho = -643 kJ/mol
C(graphite) + O2(g) -> CO2(g)
Ho = -394 kJ/mol
C(graphite) + 2H2(g) -> CH4(g)
Ho = -75 kJ/mol
2H(g) -> H2(g)
Ho = -436 kJ/mol
-804 kJ/mol
5) The standard enthalpies of formation of SO2 and SO3 are -297kJ/mol and -396kJ/mol respectively. What is the standard enthalpy of the following reaction? SO3(g) -> SO2(g) + ½O2(g)
+99 kJ/mol
6) Use the bond energies given below to estimate the change in enthalpy, H, for the following reaction: CH2CH2 + Cl2 -> ClH2C2ClH2 Bond C-C C=C C - Cl C–H Cl – Cl
Bond Energies (kJ/mol) 347 612 341 414 243
-174 kJ/mol
7) Hydrogen cyanide is produced from methane and ammonia according to the following equation: CH4(g) + NH3(g) –> HCN(g) +3H2(g) Find the enthalpy change of this reaction given the following reaction steps… H2(g) + 2C(graphite) + N2(g) –> 2HCN(g) Ho = +270. kJ N2(g) + 3H2(g) –> 2NH3(g)
Ho = -92 kJ
C(graphite) + 2H2(g) –> CH4(g)
Ho = -75 kJ
+256 kJ
8) Calculate the enthalpy change of the this reaction: 2H3BO3(aq) -> B2O3(s) + 3H2O(l), given the following reaction steps… H3BO3(aq) -> HBO2(aq) + H2O(l)
Ho = -0.02kJ
H2B4O7(s) + H2O(l) -> 4HBO2(aq)
Ho = -11.3kJ
H2B4O7(s) -> 2B2O3(s) + H2O(l)
Ho = +17.5kJ
+14.3kJ
9) The specific heat of water is 4.184 J/gC. How much heat is required to change the temperature of 42.0 g water from 22.0 C to 48.5 C?
4660 J -or- 4.66 kJ
10) In an experiment, 74.8 J of heat is required to raise the temperature of 18.69 g of silver from 10.0C to 28.0 C. What is the specific heat of silver?
0.222 J/gC
11) How much heat energy in kilojoules is required to heat all the aluminum in a roll of aluminum foil (500. grams) from room temperature, 25C to the temperature of a hot oven, 225 C? The specific heat of aluminum is 0.901 J/gC.
90.1 kJ
12) A 192g piece of copper is heated to 100.0C in a boiling water bath and then dropped into a beaker containing 750. g of water at 4.0C. If the specific heat of copper is 0.385 J/gC and the specific heat of water is 4.184 J/gC, what is the final temperature of the copper and water after they come to thermal equilibrium?
6.21C
13) Molar heat capacity is the amount of heat required to change the temperature of 1 mole of a substance by 1 C (or by 1 kelvin). Calculate the amount of heat required to change the temperature of 185.0 g of zinc from 22.5 C to 58.0 C if the molar heat capacity of zinc is 25.37 J/molC.
2,550 J -or- 2.55 kJ
14) A 0.300g sample of carbon is burned in excess oxygen in a bomb calorimeter according to the following equation: C(s) + O2(g) CO2(g) The temperature of the calorimeter increases from 25.00 C to 27.38 C. Calculate the amount of energy released during the reaction. The calorimeter has a heat capacity of 893 J/C.
-2130 J -or- -2.13 kJ
15) Calculate the enthalpy of reaction for the neutralization reaction below, given the data that follows: CsOH(aq) + HCl(aq) CsCl(aq) + H2O(l) When 100.0 mL of 0.200 M CsOH were mixed with 50.0 mL of 0.400 M HCl in a coffee-cup calorimeter, the temperature of the solutions changed from 22.500C before mixing to 24.260 C after the acidbase reaction. The densities of the solutions are 1.000 g/mL. The specific heat of the solutions are 4.150 J/gC and the heat capacity of the calorimeter is 10.30 J/C.
-1114J -or- -1.114 kJ
16) Calculate Hrxn for the combustion of ethanol according to the equation below, given the information that follows: C2H5OH(l) + 3 O2(g) 2 CO2(g) + 3 H2O(l) When a 2.84 g sample of ethanol was burned in an excess of oxygen in a bomb calorimeter, which contains 815 g of water, the temperature of the calorimeter increased from 25.00 C to 44.40 C. The specific heat of water is 4.184 J/gC and the heat capacity of the calorimeter is 923 J/C (not including the water).
-8.41 x 104 J -or- -84.1kJ
17) Calculate Hsolution when sodium nitrate dissolves in water from the following data: NaNO3(s) Na+(aq) + NO3–(aq) When 15.3 grams of NaNO3 were dissolved in 253g of water in a calorimeter, the temperature fell from 25.00 C to 21.56 C. The specific heat of water is 4.184 J/gC and the heat capacity of the calorimeter (without the water) is 12.4 J/C.
+3680 J -or- +3.68 kJ
18) Calculate the enthalpy change that occurs when 36.9 g H2S are burned according to the following equation: 2H2S(g) + 3 O2(g) 2SO2(g) + 2H2O(g)
H = -1037 kJ
-561 kJ
19) Calculate the enthalpy change that occurs when 22.4 g CaCO3 decompose according to the following equation: CaCO3(s) CaO(s) + CO2(g)
H = +178.3 kJ
+39.9 kJ
20) Calculate how many grams of natural gas (CH4) must be burned to produce 50,000. kJ of heat according to the following equation: CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g)
H = -803 kJ
999g CH4
21) What is U in Joules of a system that releases 675 J of thermal energy to its surroundings and has 2,220. Joules of work done on it?
+1545 J