Sulphate 1 1w4o50
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 2z6p3t
Overview 5o1f4z
& View Sulphate 1 as PDF for free.
More details 6z3438
- Words: 543
- Pages: 3
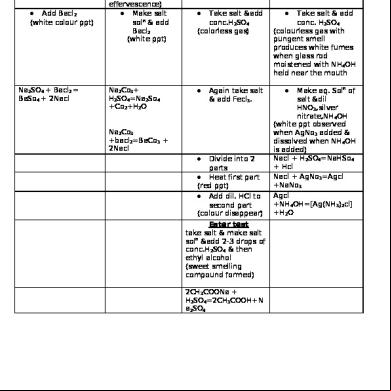
Sulphate(SO42-) •
Make salt soln
• Add Bacl2 (white colour ppt)
Na2SO4 + Bacl2 = BaSo4 + 2Nacl
Carbonate(CO32 ) •
Acetate(CH3COO-)
Chloride(Cl- )
Take salt and add dil. H2SO4 (colourless gas &brisk effervescence) • Make salt soln & add Bacl2 (white ppt)
•
Take salt and add dil. H2SO4 (no gas evolved)
•
•
•
Na2Co3+ H2SO4=Na2So4 +Co2+H2O
•
Take salt &add conc.H2SO4 (colorless gas)
Again take salt & add Fecl3.
Na2Co3 +bacl2=BaCo3 + 2Nacl •
Divide into 2 parts • Heat first part (red ppt) • Add dil. HCl to second part (colour disappear) Ester test take salt & make salt soln &add 2-3 drops of conc.H2SO4 & then ethyl alcohol (sweet smelling compound formed) 2CH3COONa + H2SO4=2CH3COOH+N a2SO4
Take salt & add dil. H2SO4 (No gas evolved)
Take salt & add conc. H2SO4 (colourless gas with pungent smell produces white fumes when glass rod moistened with NH4OH held near the mouth •
Make aq. Soln of salt &dil HNO3,silver nitrate,NH4OH (white ppt observed when AgNo3 added & dissolved when NH4OH is added) Nacl + H2SO4=NaHSo4 + Hcl Nacl + AgNo3=Agcl +NaNo3 Agcl +NH4OH=[Ag(NH3)2cl] +H2O
Ammonium(N H4+) •
Take a salt & add NaOH (pungent smell of ammonia) • Add nesslar’s Reagent (Brown ppt) NH4+ + NaOH =NH3 + H2O + Na+
Lead (Pb2+) •
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.now add dil. HCl. (white ppt) (gp 1 present) • Boil the ppt in water &filter it Divide in 3 parts Cool the firt part (white crystal of Pbcl2 appear which dissolve on heating) (Pb2+ confirmed) • Add KI to 2nd part (yellow ppt) (Pb2+ confirmed) • Add potassium chromate soln to 3rd part (yellow ppt) (Pb2+ confirmed)
Pb2+ +2HCl=Pbcl2+2H+
Pbcl2 +2KI=PbI2+2KCl
Aluminium( Al3+) •
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.now add dil. HCl. (no white ppt) (gp 1 Pb2+ absent)
•
H2S gas in soln (no coloured ppt) (gp 2 cu2+ absent) •
Add NH4cl & Nh4OH
(white gelatinous ppt observed) (gp 3 present) • Dissolve ppt in dil. NaOH & add few drops of
Strontium(Sr Barium(B 2+ ) a2+) •
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.now add dil. HCl. (no white ppt) (gp 1 Pb2+ absent
•
H2S gas in soln (no coloured ppt) (gp 2 cu2+ absent) •
Add NH4cl & NH4OH (no white ppt) 3rd gp Al3+ absent
•
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.n ow add dil. HCl. (no white ppt) (gp 1 Pb2+ absent
•
H2S gas in soln (no coloured ppt) (gp 2 cu2+ absent) • Add NH4cl & NH4O H (no white ppt) 3rd gp Al3+ absent
Calcium2+) •
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.no w add dil. HCl. (white ppt) (gp 1 Pb2+ absent
•
H2S gas in soln (no coloured ppt) (gp 2 cu2+ absent) • Add NH4cl & NH4OH (no white ppt) 3rd gp Al3+ absent
Make salt soln
• Add Bacl2 (white colour ppt)
Na2SO4 + Bacl2 = BaSo4 + 2Nacl
Carbonate(CO32 ) •
Acetate(CH3COO-)
Chloride(Cl- )
Take salt and add dil. H2SO4 (colourless gas &brisk effervescence) • Make salt soln & add Bacl2 (white ppt)
•
Take salt and add dil. H2SO4 (no gas evolved)
•
•
•
Na2Co3+ H2SO4=Na2So4 +Co2+H2O
•
Take salt &add conc.H2SO4 (colorless gas)
Again take salt & add Fecl3.
Na2Co3 +bacl2=BaCo3 + 2Nacl •
Divide into 2 parts • Heat first part (red ppt) • Add dil. HCl to second part (colour disappear) Ester test take salt & make salt soln &add 2-3 drops of conc.H2SO4 & then ethyl alcohol (sweet smelling compound formed) 2CH3COONa + H2SO4=2CH3COOH+N a2SO4
Take salt & add dil. H2SO4 (No gas evolved)
Take salt & add conc. H2SO4 (colourless gas with pungent smell produces white fumes when glass rod moistened with NH4OH held near the mouth •
Make aq. Soln of salt &dil HNO3,silver nitrate,NH4OH (white ppt observed when AgNo3 added & dissolved when NH4OH is added) Nacl + H2SO4=NaHSo4 + Hcl Nacl + AgNo3=Agcl +NaNo3 Agcl +NH4OH=[Ag(NH3)2cl] +H2O
Ammonium(N H4+) •
Take a salt & add NaOH (pungent smell of ammonia) • Add nesslar’s Reagent (Brown ppt) NH4+ + NaOH =NH3 + H2O + Na+
Lead (Pb2+) •
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.now add dil. HCl. (white ppt) (gp 1 present) • Boil the ppt in water &filter it Divide in 3 parts Cool the firt part (white crystal of Pbcl2 appear which dissolve on heating) (Pb2+ confirmed) • Add KI to 2nd part (yellow ppt) (Pb2+ confirmed) • Add potassium chromate soln to 3rd part (yellow ppt) (Pb2+ confirmed)
Pb2+ +2HCl=Pbcl2+2H+
Pbcl2 +2KI=PbI2+2KCl
Aluminium( Al3+) •
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.now add dil. HCl. (no white ppt) (gp 1 Pb2+ absent)
•
H2S gas in soln (no coloured ppt) (gp 2 cu2+ absent) •
Add NH4cl & Nh4OH
(white gelatinous ppt observed) (gp 3 present) • Dissolve ppt in dil. NaOH & add few drops of
Strontium(Sr Barium(B 2+ ) a2+) •
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.now add dil. HCl. (no white ppt) (gp 1 Pb2+ absent
•
H2S gas in soln (no coloured ppt) (gp 2 cu2+ absent) •
Add NH4cl & NH4OH (no white ppt) 3rd gp Al3+ absent
•
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.n ow add dil. HCl. (no white ppt) (gp 1 Pb2+ absent
•
H2S gas in soln (no coloured ppt) (gp 2 cu2+ absent) • Add NH4cl & NH4O H (no white ppt) 3rd gp Al3+ absent
Calcium2+) •
Take a salt & add NaOH (no brown ppt) (zero gp (NH4+) absent)
•
Take salt & make salt soln.no w add dil. HCl. (white ppt) (gp 1 Pb2+ absent
•
H2S gas in soln (no coloured ppt) (gp 2 cu2+ absent) • Add NH4cl & NH4OH (no white ppt) 3rd gp Al3+ absent