Sargent Welch Tabla 4r3q4h
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 2z6p3t
Overview 5o1f4z
& View Sargent Welch Tabla as PDF for free.
More details 6z3438
- Words: 2,191
- Pages: 2
(
(
(
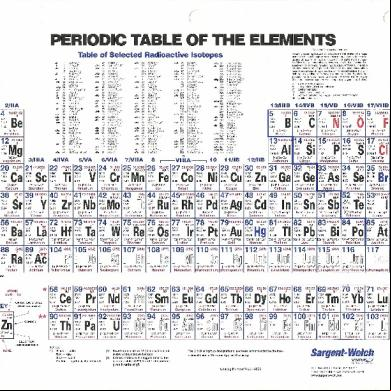
PERIODIC TABLE OF THE ELEMENTS Selected Radioactive Isotopes
Table of Selected Radioactive Isotopes ,
.•. GROUP 1/IA uNo
1 20.28 13.81 0.0899
1.00794 1,-1
"AI ••P
H ",
••s "a "k
2/11A
Hydrogen
"K
3 1615 453.7
(6.941) 1
0.534
Li [He]2s1 Lithium
1122.98~770
39.~983
Rb
87.62 2
5
5.0
39
88.9~59
V
3737 1191 6.15
Ba
K
--...
(l4,IOh1P(275dlEC (80.3d)EC
73 74 75 79 82
."
"
"'"
.s,
85 86 87 90
.Y
sa
.Rb
."
9) 95 94 95 .,Mo 99 .J, 97 911 99 ..." 106 ••Rh 101 ••Pel 103 107 •••Ag 108 110 .,Nb
•.•Xe "Cs
••Sa
,.,s,.,
(8040dl/r
15.25d)P!9.IOhjp(Hl6yj/T (2.9xlO·yl~ pO.17ylP112.80')p(6x 10'I1K [40.3hjP(2B.
tan 142 147 .,Pm 145 I"
~:~;~~~rJlp-:'
(367d) (j{3.3yJEC (170d1EC [7xlO"y\ P[127ylEC f252d]p-
146 151 152
1140~EC (7S.1diP-
.,Po 231
p.26J<.10'l1o
..,u
(L59x IOJrlo /2.44><10'ylex 17.04~ IO"yla /2.34)( IO'\'io. (4.£7~ 10·y!0. (llxlO'yjEC Ir f2:'4x 10'yla' f2.346dJp[8715ylo (2.41 x 10'ylo. /6.54xlO'y)a (3.8" 10'ylo: [8.3x 10'yja j432yla 17.37xIO'y)1l !!63.2d)1l (18.12ylo {1.55x 10'110 (3.5x 10'yJ c, Sf (lAx 10'yjll (35lylo !'lOOnW2d)a
233
23< 235 236
aaa .,N:p236
...p,.
""
.,Eu (13yJP"',EC,p(8.5yI P•• Gel 150 (2.1 x W'yla 158 !1.2~ 10'ylEC,lr 160 (72.3dJP.,Ho 166mll.2x 10'yJ tr ••Tm 170 1128.6d)iJ171 {l.92)jtr ",Yb 169 [32.00')EC (4.19d)p175 "Lu 176 [3.7><10'°yjp"To 182 (115.0d)/l-
'"
'"
2J9 238 239 2"
'"
2M ••Am
••em
241 2<1 2t;2
,..
'"
..... ""
(93yjp-
•• 1b
109 [453d)EC 11~ (A9.51djlT 121 (76ylp-
..In
131
1M
.,Sm
,.lIe ,.Os
133 135 13~ 135 137 UO 137
17W
uCc •• Pr ",Nd
(7.45~/r
III
..cd
>.>1
[17.9d)P-,jl",K [118.5d)P(6.5xIO'yl/T [35.34hIP(2.1x lO'ylEC (lO.72ylfr [l8.7djP[4.8xl0"ylP{28.8y1P(I06.6dl/l",EC 11.5x lO'ylP{MOdjP{2.0xIO'}'lP(35.15d)jJ (66.02hl/r {2.6xI0·yJEC
181 185 188 167 194
[69djll(5~ IO'°ylP(6.0)'1 pmr 192 (74.2diP-,P+.EC rtAu 195 (163~EC 196 [6.18dJP",£(,P19' (2.696d)P199 [3.15dJf1oHg 203 [46.8d)P••n (3.77yjtr "Pb 202 [3xlO'ylEC 205 [h 10'ylEC 210 (22.3ylfF,a .,Bi 207 (38ylEC 206 (3.7xlO'}'lEC 210 (5.Old)r,o 2!Om{3x 10"yla (2.90ylll 20> [I02yla 210 (138.38d]0: uAI 20> (5.4I1EC,a 210 (El.1hjEC 211 (7.2IhjK,0 ••Rn222 (3.824dJa .,f.212 {19.311"1i1i EC.a 221 [I5minlr P22J (21.811"1i1i uRa 226 [L60x 10'yl a (2I.77I1P•• Ac 227 ••Th228 (1.913\1'" 230 a 232
m(154d!rr 123m(119.7d)IT 117m(I09d)fT 129 (1(nlO\·ilf"
{78.2hlEC
68
"'"
Ra [Rn)7s2 Radium
89
*
A**
178.49 4
72 4876 2506
Hf
13.31
[Xe]4f14Sd26s2 Hafnium
J227)
3470· 1324 10.07
Zr
SNA 23 3680 2183
C
[Rn)6dt7s2 Actinium
104
-
51.996 3,6,2
24 2945 2180
Cr [Ar]3d54s1 Chromium
4912M 2896
8.57
73'80.9:,~9
~~
W
19.3
Ta
[Xe]4f145d36s2 Tantalum
-
183.84 6,5,4,3,2
106
~~
{Rn]5ft46d37s2' Dubnium
26 3134 1811
2" ••8k2A7 ••CJ
2A9
251 252 253 2" \oofm255 257 ,o,Md258 \00""0259 ,o..d.r260 ,•.•Rf261 ,•••Db262 ,ooSg263
ooE5
75 21.0
~~
-
07
101.07 2,3,4,6,8
44 4423 2607
R
U
12.37
22.57
58.~:;2
3186 1728
p+ EC IT
S,
13/111B 14/1VB
"'<-
14040 p.9~0:
8.90
8.90 Ni [Ar]3de4s2 Nickel
Co [Ar)3d74s2 Cobalt
451
1
~~
-
02.92?;'~0
3968 2237 12.41
Rh
0S 77
19~::,g
78
4700 2720
22.42
08~277)
~~
(Rnj5f'46d67s2' Hassium
Ir
79969~,~55
P
3130 A 1337.33 19.3
t
[Xe]4f'4Sd96s1 Platinum
09~268)
-
11
0~69)
'4
I~
10'0 Cd 594.22 8.65 [Kr]4d1°5s2 Cium 200.59 2.1
80
U
62988 234.32
(272)
[Xe]4f'4Sd106s2 Mercury 112
-
~~
-
[An]5f'46d87s2· (Darmstadtium)
12.0107 ±4,2
8 90.2 54.4
15.9994 ·2,·1
0
1.429t [He]2s22p4 Oxygen
1326.98;538
14
1530_9::~~
2792 933.5
3538 1687
553 317.3
16 717.75 388.36
2.33
1.82
28.~,~55
5i [Ne]3s23p2 Silicon
[Ne]3s23pt Aluminum 69.723 1,3
31
a
2477 G 302.91 6.095
114.82 3
e
3106 G 1211.4 5.32
[Ar]3d'04s24pl Gallium 49 2345 429.75
72.64 4
32
[Ar]3d104s24p2 Germanium
50 1181.~0
n
2875 5 505.08
In 7.31 [Kr)4dlOSs2Spl Indium 81204.3:~3
7.31 [Kr]4dlOSs2Sp2 Tin
207.2 2,4
82
1746 577
2022 600.61
Pb
11.85 11.35 TI [Xe)4f14Sd1Of3s26pl [XeJ4P45dt06s26p2 Thallium Lead 113
(284)
114
P
(289)
33
5
,l1~';;,
5.73 [Ar]3d104s24p3 Arsenic 121.760 t3,5
51 186°5b 903.78
239.11 171.65
78.96 4,6,-2
83208.9:~4
84
1837 544.55
-
127.60 4,6,-2
(288)
35.453 :t1,7.5,3
18
CI
87.8 83.8
(209) 4,2,6
{Ne]3s23p6 Argon
9.3 Po (Xe]4f'45dtOBs26p4 Polonium 116
36 119,93 115.8 3.73t
53126.;~~:;7
54
Br {Ar]3d104s24p5 Bromine
I
4.93
[Kr]4d1OSs2Sp5 Iodine
85 610" 575
A
1.784t
79.904 ±t,7,5,3
(210) ±1,7,5,3
ATOMIC
30 1180. 69273
**
n
Z
58
140.~,:6
3716 1071 6.77
Ce
[Xe)4f1Sd'6s2 Cerium
/ DENSITY at 300 K (3) (g/cm3) ©Copyrighl2007 WVR International. All Rights Reserved. No portion of this work may be reproduced in any form or by any means wittnrt express prior written permission from VWRlSargentWelch.
\ NAME
ELECTRON CONFIGURATION
7.01
Pr
92
912310~;9
5061 2023
4300' 1645
4404 1406
15.4'
16.9S
Th
Pa (Rn]5f26d17s2 Protactinium
NOTES: (1) Btack - solid. Red - gas. Blue - liquid. Outline - synthetically
23:5~4~:,;
U
[Rn)S/36d17s2 Uranium
(2) (3) prepared.
61 3273 1315 7.26
Nd
[Xe]4f46s2 Neodymium
[Xe]4f36s2 Praseodymium
90232.03:1
IRn)8d27s2 Thorium
Zinc
6.77
144.24 3
60 3347 1294
3785 1204
11.72 7.1~ArJ3d1Q4S2
59140.9~~65
(145) 3
20.2
(237) 5,6,4,3
63
151:~4
2007 1347
1869 1095
7.S2 5 [Xe]4f66s2 Samarium
5.24
~I m
[Xe]4f56s2 Promethium 93 4175' 917
150.36 3,2
62
94 3505 913
(244) 4,5,5,3
3546 1586
65158.9~,~34
8.23
[Xe]4FSd'6s2 Gadolinium
(243) 3,6,5,4,2
(247) 3
96
-
66 2840 1685
3503 1629
Gd
7.90
Eu
jXej4176s2 Europium 95 226<1 1449
157.25 3
64
8.55
Tb
[Xe]4f96s2 Terbium (247) 2,3,4
97
1620
-
13.S"
14'
162.50 3
Dy
19.84
[Rn]Sf&7s2 Plutonium
Based upon carbon-tz. () indicates most stable or best known isotope. Entries marked with daggers refer to the qaseous state at 273 K and 1 atm and are given in units of gli.
13.7
{Rn]SF7s2 Americium
[Rn)Sf16d'7s2 Curium
The A & B subgroup national
(251) 2,3,4
designations,
99
1130·
-
~~
{An]5fl07s2 Californium
are those
recommended
Chemistry.
68 3140 1802 9.07
-
(252) 3
~~
[Rn)5f117s2 Einsteinium
by the Inter-
167.26 3
Kr [Ar]3d104s24p6 Krypton
.Xie 131.29 0,2,4,6,8
165.11 161.4 5.90t
[Kr]4d105s2Sp5 Xenon (222) 0,2
86 211.4R 202
-
118
117
~M~ ~M~
[Rn]Sf146d1Q7slJp4 (Ununhexium) (Ununseptium)
69168.;~:2
70
Er
100
-
(;57)
101
(;58)
-
[Rn]Sf'27s2 Fermium
[Rn]5t137s2 Mendelevium
71 3675 1936
(Ununoctium)
102
-
1100·
-
Lu [Xe]4ft45dI6s2 lutetium
(2~';)
oo@
[Rn]St 147s2 Nobelium
1
03
1900'
-
WL$·18806
~~
(Rn]Sf'46dt7s2 Lawrencium
Sargent-Weich INlEflNATIONAl
Number
(2~2)
-
vWR'-b~ Cataloq
174.967 3
9.841
[Xe]4f146s2 Ytterbium
~I I~ 1100'
Vb
6.903
[Xe)4f'36s2 Thulium
-
1800'
-
m
173.04 3,2
1469 1092
P.O. Box 4130 • Buffalo, NY 14217 1-800-727-4368· FAX 1·800-676-2540 www.sargenlwelch.com
n
9.73t At [Xe)4f14Sd'OSs26p5 [Xe]4f'45dl06s26p Radon Astatine
[Rn]5f'~6d'07s27p3· (Ununpentium)
2223T 1818 9.32
r
83.80 0,2
-
[Xe]4ft26s2 Erbium
[Xe]4fI16s2 Holmium
1170·
[Rn]Sf97s2 Berkelium
Union of Pure and Applied
8.795
-
OO~ ~M ~I ~I ~lli
[Rn]Sf46d17s2 Neptunium
2973 1747
[Xe]4f'06s2 Dysprosium 98
H0
67'64.9;°3
e
39.948
3.12
(Kr]4dtOSs2Sp4 Tellurium
527
115
0.900t [He]2s22pO Neon
[Ne]3s23p5 Chlorine 35 331.95 265.95
20.1797
27.07N 24.56
457.51 386.85 Te
tsa Helium 10
3.214t
5e [Ar]3d'04s24p4 Selenium
52 1261 722.66
18.99840 ·1
F 1.696t [He]2s22pS Fluorine
4.79
6.24
9.75 Bi {Xe]4ft45d'06s26p3 Bismuth
9 85.0 53.55
32.065 6,:1:2,4, 17
34 958 494
He
17NIIB
5 [Nej3s23p4 Sulfur
6.69 [Kr]4d tOSs25p3 Antimony
==
[Rn[SP46<:PO]s27pl' ~'151"~~77~ (Ununtrium) (Ununquadium)
74';i~6
876'-'A
4.00260
216 4. 0.95 al26atm 0.1785t
2.07
[Ne]3s23p3 Phosphorus
~M~ ~M~
[Rn]Sf146dt07s2' (Ununbium)
14.0067 ±3,5,4,2
7 77.344 63.15
N 1.251t [He]2s22p3 Nitrogen
AI
18NIII 2
16NIB
1SNB
-
~~~ -
[AnJ5f146d97s (Roentgenium)
(285)
6 4675· 3915·
-
-
-
H9
13.55
j
11
112.41 2
48
[Xe]4f'4Sd106s1 Gold
-
-
[RnjSf 6d77s2' Meitnerium
1180 692.66 7.13
[Kr]4d105s' Silver
195.08 4,2
4098 2041.55 21.45
(Xe]4fI4Sd76s2 Iridium
1
-
10.50
Pd
[Kr]4d'O Palladium
Zn 65.409 2
30
[Arj3d'04s2 Zinc
9
2435 A 1234.93
12.0
[Kr]4d6Ss' Rhodium
47107.8,682
by a mass
2.26 C [He]2s22p2 Carbon
12/11B
[Arj3d'04s' Copper
106.42 2,4
46 3236 1828
U
C
are designated
B 2.37 [He]2s22p1 Boron
2.6989
63.546 2,1
2835 1357.8 8.96
10.811 3
5 4275 2348
f20.AldJa
29
isotopes
alpha particle emission beta particle (electron) emission positron emission orbital electron capture isomerictransilion from upper to lower isomeric state spontaneous fission
" rr
11/1B
28586i;4
3200 1768
IXe]4f'45d66s2 Osmium
~264)
IRnj5t'46d57sZ' Bohrium
27
190.23 4,8,6,3,2
76
10
VIIIA-----,
[Kr]4d7Ss' Ruthenium
5285 3300 Re
55.845 3,2,6
Fe [Ar}3d54s2 Iron
(Xe]4f'45ds6s2 Rhenium 1
[Rn)Sj146d47s2· Seaborgium
!,~~5;?;2
sa70 3459
.----
radioactive
Half-lives follow in parentheses, where e. min. h. d, and y stand respectivefyforseconds, minutes, hours, days, and years. The table includes mainly the longer-lived radioactive isotopes; many curers have been prepared. Isotopes known to be radioactive but wilh halt-fives exceeding lO'~ y have not been included. Symbols describing Ihe principal mode (or modes) of decay are as follows (theseprocessesaregenerallyaccompaniedbygammaradialion)'
f276dJo (20.1hja (IOO5dJo {55d)a {58",.,0: p.O monj 0
~~:;;~~~rl~
7.874
[Krj4d55s2 Technetium
(266)
-
-
¥©
11.5"
[Xe]4f'45d46s2 Tungsten
05~262)
(98) 7,5,4
43 4538 2430
{Kr]4d5Ss' Molybdenum 74 5828 3695
5730 3290 16.65
0
10.22
Nb
[Kr]4d4Ssl Niobium
n
5~;,~~?7
8
[Ar]3d54s2 Manganese
95.94 6,5,4,3,2
42
5017 2730
25 2234M 1519 7.44
7.19
4192.90;,~,~
1
[Rn)5ft46d27s2' Rutherfordiurn
V
7NIIA
6NIA
[Ar)3d34s2 Vanadium
~61)
-
5°·:~1:2
6.11
[Kr)4d2Ss2 Zirconium
a
L
91.224 4
40 4682 2128
[Xe]5d16s2 Lanthanum (226) 2
47.867 4,3,2
6.51
POINT, K
'
4/1VA
{Kr]4d15s2 Yttrium
* BOlu::MBE\R KE~Ej'GHTO;;~~T!~~1 ~:,;,~S POINT
(6L88hllr
[2M.ldiP+,E(
••w
(W.20d)/r (2.7)'!P-
in blue (although some are also rnanutactueeo). Letter m inan isomer 01 anomer isotope of the same mass number
Values
ATOMIC
MELTlN~
•."
4.54 Ti {Ar]3d24s2 Titanium
5c [Ar]3d'4s2 Scandium
57138.9~55
88 1413 973
Fr
• Estimated
2.99
[Xe]6s2 Barium
[Rn]7st Francium
(78.8d)P+,EC (270djEC (71.3d]p•.,EC 15·272Ylr (36.0h!P+,EC [8xlO'I1K (92YlP(12.70hlP-, p ". EC
22 3560 1941
4.47
5r
3.5
(223) 1
-
2144.95,591
3618 1795
2170 1000
[Xej6s1 Cesium 87 950' 300
3/11IA
56137}27
C
uN.
56 57 58 60 57
"""
[Kr)Ss2 Strontium
55132.9?545
(44.6d]{r
6)
3109 1814
2.54
.,Ge :uAs
67
65 67 72
124 125 121
number dicates
9
40.078 2
38 1655 1050
[Kr)Ssl Rubidium
944 301,54 1.87
9
Ca [Ar]4s2 Calcium
1.532
ss
,,<-
1.55
K
85.4~78
312.46 961
"
.Fe
24.;050
20 1757 1115
[Ar]4s' Potassium 37
,,1M
1363M 923 1.74 [Ne]3s2 Magnesium
1033 336.8 0.862
"c,
12
[Ne]3s' Sodium 19
,,"
Be [He]2s2 Beryllium
",In .,Go
"'"
.eo
1.85
a
1156.1N 371.0 0.971
9.012182 2
4 2744 1560
m
"
"Mg
t
P5_3 "ilr
3 1l2.26yl/r 7 (53.3diEC 10 1'.6~ IO'yl Ill' 12040m"ip+ 14 (5730Yllr 18 1l09_B~r 22 [2.602ylP,EC [I5.02,*P28 [20.9hItr 26 [7.2x IO'ylf3~,EC 32 II4.26d)p35 (87.2d) {r 36 13.01x IO'ylP36 (37.2m~p37 (35.Q2d]K 39 1265}'ltr (L28x 10'ylEC '0 112.36I1P(165d)pao 183.80d)P51 (27.70d)EC 53 (2xI0"I1EC (313.0d)EC 56 (2.578h1lr
,H
.,Sb
Naturally occurring
Side 1
TABLE OF PERIODIC PROPERTIES OF THE ELEMENTS Percent Ionic Character of a Single Chemical Bond Difference
GROUP
in electronegativity
0.1
0.20.3
0.4
0.5 0.6
0.7
0.8
0.9
1.0
1.1
1.2
1.3
1.4
1.5
1.6
1.7
1.8 1.92.0
2.1
2.2
2A
2.3
2.5
2.62.72.8
2.9
3.0
3.1
3.2
18/V1II
:L/IA Percentionicchoeccree 0.32 0.79 14.10 13.598 14.304
2.20 0.4581 0.0585 0.1815
%
DATA CONCERNING
0.5 1
2
6
9
12 '5
Neutron
Electron'
Proton
Symbol
e
P
Rest moss
1,67495)(10.27 1.67265x10·27
(kg)
Relativeotomiemoss ("C:12)
19 22 26 30 34 39 43 47 51 55 59 63 67 70 74 76 79 82 84 86 88 89 91 92
THE MORE STABLE ELEMENTARY (SUBATOMIC)
2/IIA
Charge
4
1.008665
(e)
Neutrino
9.1095xI0·31
~O
1.007276
5,48580xl 0.4
~o
1.60219x10·19
.1.60219)(10.19
1/2
1/2
-1,913JIN
Magnetic Momentt
CRYSTAL STRUCTURE
• The positron {e+] has properties similar to those of the (neg· ative) ele
photon
-0 1/2
Spin quantum number
2.793
0.93 0.49 31.80 24.587 5.193
PARTICLES
(e-)
Radius (m)
He@ 0.084 0.021 0.152
tJlB:Bohr mogneton ond J.IN=Nudeor magneton,
1/2
1.001 JIB
JiN
ACIO·BASE (2)
PROPERTIES
(I)
ELECTRONEGATIVITY,
(Pauling's)
SYMBOL
COVALENT
ATOMIC
RADIUS,
VA~~:I~~~ON kJ fmol (4)
A (7)
RADIUS, ATOMIC
HEAT
VOLUME,
OF FUSION
kJ/mol
cm'/mol{8)
FIRST
ELECTRICAL
IONIZATION
©Copyright
1992
15.0 5.89
o'n' m'
SPECIFIC HEAT CAPACITY, Jg" K' (3)
THERMAL
12.50 6.194 0.12
5.6 47
21.10 6.266
12.32 6.026 0.13'
0.7 6.74
valence)
of group.
Oxide
shown.
Intensity
0.8 6.3
20.8 5.974
18.3 5.991
0.7 10'
6.198
6.282
10'
10'
6.42 10'
6.5 10'
6.58 10'
6.65 10'
4.9'
to-
10'
(5)
(1) For representative and
(6)
(2)
@Copyrightl993
©Copyrighl1994 @Copyrighl1995 ©Copyright 1996 ©Copyright 1998 @Copyrighl2000 @CopyrighI2001 ©Copyrighl2002 @ Copyrighl 2004 e Copyrighl 2007
1.3 543.92 15.65 7.1 54
CONDUCTIVITY 1
V 1979 1980
19.90 6.31 0.113
NOTES:
POTENTIAL
e Copyright ©Copyright
**
A
CONDUCTIVITY, Wm·'K·'(3)
amphoteric
* ~
oxides if both
Cubic, face hexagonal;
(higher
colors
are
centered;
~
cubic,
@
rhombohedral;
(6)
Generally
body
LD
is acidic
e
af color indicates
centered;
UI
tetragonal;
if color
is red,
relative
basic
if color
is blue
strength. The A & B subgroup designations, are those recommended by the International Union of Pure and Applied Chemistry,
cubic;
orthorhombic;
0
manoclinic.
Sargent-Welch VWR'-J" INHRNAT10NAL
(3)
At 300 K (>7'C)
(4)
At boiling
(5)
At melting
point point
at 293
K (20°C)
for polycrystalline (7)
Quantum for frcc
mechanical
material value
(8)
From density and refer
at 300
solid clements: to liquid
state
K (27°C) for liquid values
for gaseous
at boiling
PO. Box 4130· Buffalo, NY 14217 1-800·727-4368· FAX 1-800-676-2540 www.sargenlwelch.com
elements
point
atom Catalog
Number
WLS·18806
SIDE
2
VVVR Intemalional
)
)
)
(
(
PERIODIC TABLE OF THE ELEMENTS Selected Radioactive Isotopes
Table of Selected Radioactive Isotopes ,
.•. GROUP 1/IA uNo
1 20.28 13.81 0.0899
1.00794 1,-1
"AI ••P
H ",
••s "a "k
2/11A
Hydrogen
"K
3 1615 453.7
(6.941) 1
0.534
Li [He]2s1 Lithium
1122.98~770
39.~983
Rb
87.62 2
5
5.0
39
88.9~59
V
3737 1191 6.15
Ba
K
--...
(l4,IOh1P(275dlEC (80.3d)EC
73 74 75 79 82
."
"
"'"
.s,
85 86 87 90
.Y
sa
.Rb
."
9) 95 94 95 .,Mo 99 .J, 97 911 99 ..." 106 ••Rh 101 ••Pel 103 107 •••Ag 108 110 .,Nb
•.•Xe "Cs
••Sa
,.,s,.,
(8040dl/r
15.25d)P!9.IOhjp(Hl6yj/T (2.9xlO·yl~ pO.17ylP112.80')p(6x 10'I1K [40.3hjP(2B.
tan 142 147 .,Pm 145 I"
~:~;~~~rJlp-:'
(367d) (j{3.3yJEC (170d1EC [7xlO"y\ P[127ylEC f252d]p-
146 151 152
1140~EC (7S.1diP-
.,Po 231
p.26J<.10'l1o
..,u
(L59x IOJrlo /2.44><10'ylex 17.04~ IO"yla /2.34)( IO'\'io. (4.£7~ 10·y!0. (llxlO'yjEC Ir f2:'4x 10'yla' f2.346dJp[8715ylo (2.41 x 10'ylo. /6.54xlO'y)a (3.8" 10'ylo: [8.3x 10'yja j432yla 17.37xIO'y)1l !!63.2d)1l (18.12ylo {1.55x 10'110 (3.5x 10'yJ c, Sf (lAx 10'yjll (35lylo !'lOOnW2d)a
233
23< 235 236
aaa .,N:p236
...p,.
""
.,Eu (13yJP"',EC,p(8.5yI P•• Gel 150 (2.1 x W'yla 158 !1.2~ 10'ylEC,lr 160 (72.3dJP.,Ho 166mll.2x 10'yJ tr ••Tm 170 1128.6d)iJ171 {l.92)jtr ",Yb 169 [32.00')EC (4.19d)p175 "Lu 176 [3.7><10'°yjp"To 182 (115.0d)/l-
'"
'"
2J9 238 239 2"
'"
2M ••Am
••em
241 2<1 2t;2
,..
'"
..... ""
(93yjp-
•• 1b
109 [453d)EC 11~ (A9.51djlT 121 (76ylp-
..In
131
1M
.,Sm
,.lIe ,.Os
133 135 13~ 135 137 UO 137
17W
uCc •• Pr ",Nd
(7.45~/r
III
..cd
>.>1
[17.9d)P-,jl",K [118.5d)P(6.5xIO'yl/T [35.34hIP(2.1x lO'ylEC (lO.72ylfr [l8.7djP[4.8xl0"ylP{28.8y1P(I06.6dl/l",EC 11.5x lO'ylP{MOdjP{2.0xIO'}'lP(35.15d)jJ (66.02hl/r {2.6xI0·yJEC
181 185 188 167 194
[69djll(5~ IO'°ylP(6.0)'1 pmr 192 (74.2diP-,P+.EC rtAu 195 (163~EC 196 [6.18dJP",£(,P19' (2.696d)P199 [3.15dJf1oHg 203 [46.8d)P••n (3.77yjtr "Pb 202 [3xlO'ylEC 205 [h 10'ylEC 210 (22.3ylfF,a .,Bi 207 (38ylEC 206 (3.7xlO'}'lEC 210 (5.Old)r,o 2!Om{3x 10"yla (2.90ylll 20> [I02yla 210 (138.38d]0: uAI 20> (5.4I1EC,a 210 (El.1hjEC 211 (7.2IhjK,0 ••Rn222 (3.824dJa .,f.212 {19.311"1i1i EC.a 221 [I5minlr P22J (21.811"1i1i uRa 226 [L60x 10'yl a (2I.77I1P•• Ac 227 ••Th228 (1.913\1'" 230 a 232
m(154d!rr 123m(119.7d)IT 117m(I09d)fT 129 (1(nlO\·ilf"
{78.2hlEC
68
"'"
Ra [Rn)7s2 Radium
89
*
A**
178.49 4
72 4876 2506
Hf
13.31
[Xe]4f14Sd26s2 Hafnium
J227)
3470· 1324 10.07
Zr
SNA 23 3680 2183
C
[Rn)6dt7s2 Actinium
104
-
51.996 3,6,2
24 2945 2180
Cr [Ar]3d54s1 Chromium
4912M 2896
8.57
73'80.9:,~9
~~
W
19.3
Ta
[Xe]4f145d36s2 Tantalum
-
183.84 6,5,4,3,2
106
~~
{Rn]5ft46d37s2' Dubnium
26 3134 1811
2" ••8k2A7 ••CJ
2A9
251 252 253 2" \oofm255 257 ,o,Md258 \00""0259 ,o..d.r260 ,•.•Rf261 ,•••Db262 ,ooSg263
ooE5
75 21.0
~~
-
07
101.07 2,3,4,6,8
44 4423 2607
R
U
12.37
22.57
58.~:;2
3186 1728
p+ EC IT
S,
13/111B 14/1VB
"'<-
14040 p.9~0:
8.90
8.90 Ni [Ar]3de4s2 Nickel
Co [Ar)3d74s2 Cobalt
451
1
~~
-
02.92?;'~0
3968 2237 12.41
Rh
0S 77
19~::,g
78
4700 2720
22.42
08~277)
~~
(Rnj5f'46d67s2' Hassium
Ir
79969~,~55
P
3130 A 1337.33 19.3
t
[Xe]4f'4Sd96s1 Platinum
09~268)
-
11
0~69)
'4
I~
10'0 Cd 594.22 8.65 [Kr]4d1°5s2 Cium 200.59 2.1
80
U
62988 234.32
(272)
[Xe]4f'4Sd106s2 Mercury 112
-
~~
-
[An]5f'46d87s2· (Darmstadtium)
12.0107 ±4,2
8 90.2 54.4
15.9994 ·2,·1
0
1.429t [He]2s22p4 Oxygen
1326.98;538
14
1530_9::~~
2792 933.5
3538 1687
553 317.3
16 717.75 388.36
2.33
1.82
28.~,~55
5i [Ne]3s23p2 Silicon
[Ne]3s23pt Aluminum 69.723 1,3
31
a
2477 G 302.91 6.095
114.82 3
e
3106 G 1211.4 5.32
[Ar]3d'04s24pl Gallium 49 2345 429.75
72.64 4
32
[Ar]3d104s24p2 Germanium
50 1181.~0
n
2875 5 505.08
In 7.31 [Kr)4dlOSs2Spl Indium 81204.3:~3
7.31 [Kr]4dlOSs2Sp2 Tin
207.2 2,4
82
1746 577
2022 600.61
Pb
11.85 11.35 TI [Xe)4f14Sd1Of3s26pl [XeJ4P45dt06s26p2 Thallium Lead 113
(284)
114
P
(289)
33
5
,l1~';;,
5.73 [Ar]3d104s24p3 Arsenic 121.760 t3,5
51 186°5b 903.78
239.11 171.65
78.96 4,6,-2
83208.9:~4
84
1837 544.55
-
127.60 4,6,-2
(288)
35.453 :t1,7.5,3
18
CI
87.8 83.8
(209) 4,2,6
{Ne]3s23p6 Argon
9.3 Po (Xe]4f'45dtOBs26p4 Polonium 116
36 119,93 115.8 3.73t
53126.;~~:;7
54
Br {Ar]3d104s24p5 Bromine
I
4.93
[Kr]4d1OSs2Sp5 Iodine
85 610" 575
A
1.784t
79.904 ±t,7,5,3
(210) ±1,7,5,3
ATOMIC
30 1180. 69273
**
n
Z
58
140.~,:6
3716 1071 6.77
Ce
[Xe)4f1Sd'6s2 Cerium
/ DENSITY at 300 K (3) (g/cm3) ©Copyrighl2007 WVR International. All Rights Reserved. No portion of this work may be reproduced in any form or by any means wittnrt express prior written permission from VWRlSargentWelch.
\ NAME
ELECTRON CONFIGURATION
7.01
Pr
92
912310~;9
5061 2023
4300' 1645
4404 1406
15.4'
16.9S
Th
Pa (Rn]5f26d17s2 Protactinium
NOTES: (1) Btack - solid. Red - gas. Blue - liquid. Outline - synthetically
23:5~4~:,;
U
[Rn)S/36d17s2 Uranium
(2) (3) prepared.
61 3273 1315 7.26
Nd
[Xe]4f46s2 Neodymium
[Xe]4f36s2 Praseodymium
90232.03:1
IRn)8d27s2 Thorium
Zinc
6.77
144.24 3
60 3347 1294
3785 1204
11.72 7.1~ArJ3d1Q4S2
59140.9~~65
(145) 3
20.2
(237) 5,6,4,3
63
151:~4
2007 1347
1869 1095
7.S2 5 [Xe]4f66s2 Samarium
5.24
~I m
[Xe]4f56s2 Promethium 93 4175' 917
150.36 3,2
62
94 3505 913
(244) 4,5,5,3
3546 1586
65158.9~,~34
8.23
[Xe]4FSd'6s2 Gadolinium
(243) 3,6,5,4,2
(247) 3
96
-
66 2840 1685
3503 1629
Gd
7.90
Eu
jXej4176s2 Europium 95 226<1 1449
157.25 3
64
8.55
Tb
[Xe]4f96s2 Terbium (247) 2,3,4
97
1620
-
13.S"
14'
162.50 3
Dy
19.84
[Rn]Sf&7s2 Plutonium
Based upon carbon-tz. () indicates most stable or best known isotope. Entries marked with daggers refer to the qaseous state at 273 K and 1 atm and are given in units of gli.
13.7
{Rn]SF7s2 Americium
[Rn)Sf16d'7s2 Curium
The A & B subgroup national
(251) 2,3,4
designations,
99
1130·
-
~~
{An]5fl07s2 Californium
are those
recommended
Chemistry.
68 3140 1802 9.07
-
(252) 3
~~
[Rn)5f117s2 Einsteinium
by the Inter-
167.26 3
Kr [Ar]3d104s24p6 Krypton
.Xie 131.29 0,2,4,6,8
165.11 161.4 5.90t
[Kr]4d105s2Sp5 Xenon (222) 0,2
86 211.4R 202
-
118
117
~M~ ~M~
[Rn]Sf146d1Q7slJp4 (Ununhexium) (Ununseptium)
69168.;~:2
70
Er
100
-
(;57)
101
(;58)
-
[Rn]Sf'27s2 Fermium
[Rn]5t137s2 Mendelevium
71 3675 1936
(Ununoctium)
102
-
1100·
-
Lu [Xe]4ft45dI6s2 lutetium
(2~';)
oo@
[Rn]St 147s2 Nobelium
1
03
1900'
-
WL$·18806
~~
(Rn]Sf'46dt7s2 Lawrencium
Sargent-Weich INlEflNATIONAl
Number
(2~2)
-
vWR'-b~ Cataloq
174.967 3
9.841
[Xe]4f146s2 Ytterbium
~I I~ 1100'
Vb
6.903
[Xe)4f'36s2 Thulium
-
1800'
-
m
173.04 3,2
1469 1092
P.O. Box 4130 • Buffalo, NY 14217 1-800-727-4368· FAX 1·800-676-2540 www.sargenlwelch.com
n
9.73t At [Xe)4f14Sd'OSs26p5 [Xe]4f'45dl06s26p Radon Astatine
[Rn]5f'~6d'07s27p3· (Ununpentium)
2223T 1818 9.32
r
83.80 0,2
-
[Xe]4ft26s2 Erbium
[Xe]4fI16s2 Holmium
1170·
[Rn]Sf97s2 Berkelium
Union of Pure and Applied
8.795
-
OO~ ~M ~I ~I ~lli
[Rn]Sf46d17s2 Neptunium
2973 1747
[Xe]4f'06s2 Dysprosium 98
H0
67'64.9;°3
e
39.948
3.12
(Kr]4dtOSs2Sp4 Tellurium
527
115
0.900t [He]2s22pO Neon
[Ne]3s23p5 Chlorine 35 331.95 265.95
20.1797
27.07N 24.56
457.51 386.85 Te
tsa Helium 10
3.214t
5e [Ar]3d'04s24p4 Selenium
52 1261 722.66
18.99840 ·1
F 1.696t [He]2s22pS Fluorine
4.79
6.24
9.75 Bi {Xe]4ft45d'06s26p3 Bismuth
9 85.0 53.55
32.065 6,:1:2,4, 17
34 958 494
He
17NIIB
5 [Nej3s23p4 Sulfur
6.69 [Kr]4d tOSs25p3 Antimony
==
[Rn[SP46<:PO]s27pl' ~'151"~~77~ (Ununtrium) (Ununquadium)
74';i~6
876'-'A
4.00260
216 4. 0.95 al26atm 0.1785t
2.07
[Ne]3s23p3 Phosphorus
~M~ ~M~
[Rn]Sf146dt07s2' (Ununbium)
14.0067 ±3,5,4,2
7 77.344 63.15
N 1.251t [He]2s22p3 Nitrogen
AI
18NIII 2
16NIB
1SNB
-
~~~ -
[AnJ5f146d97s (Roentgenium)
(285)
6 4675· 3915·
-
-
-
H9
13.55
j
11
112.41 2
48
[Xe]4f'4Sd106s1 Gold
-
-
[RnjSf 6d77s2' Meitnerium
1180 692.66 7.13
[Kr]4d105s' Silver
195.08 4,2
4098 2041.55 21.45
(Xe]4fI4Sd76s2 Iridium
1
-
10.50
Pd
[Kr]4d'O Palladium
Zn 65.409 2
30
[Arj3d'04s2 Zinc
9
2435 A 1234.93
12.0
[Kr]4d6Ss' Rhodium
47107.8,682
by a mass
2.26 C [He]2s22p2 Carbon
12/11B
[Arj3d'04s' Copper
106.42 2,4
46 3236 1828
U
C
are designated
B 2.37 [He]2s22p1 Boron
2.6989
63.546 2,1
2835 1357.8 8.96
10.811 3
5 4275 2348
f20.AldJa
29
isotopes
alpha particle emission beta particle (electron) emission positron emission orbital electron capture isomerictransilion from upper to lower isomeric state spontaneous fission
" rr
11/1B
28586i;4
3200 1768
IXe]4f'45d66s2 Osmium
~264)
IRnj5t'46d57sZ' Bohrium
27
190.23 4,8,6,3,2
76
10
VIIIA-----,
[Kr]4d7Ss' Ruthenium
5285 3300 Re
55.845 3,2,6
Fe [Ar}3d54s2 Iron
(Xe]4f'45ds6s2 Rhenium 1
[Rn)Sj146d47s2· Seaborgium
!,~~5;?;2
sa70 3459
.----
radioactive
Half-lives follow in parentheses, where e. min. h. d, and y stand respectivefyforseconds, minutes, hours, days, and years. The table includes mainly the longer-lived radioactive isotopes; many curers have been prepared. Isotopes known to be radioactive but wilh halt-fives exceeding lO'~ y have not been included. Symbols describing Ihe principal mode (or modes) of decay are as follows (theseprocessesaregenerallyaccompaniedbygammaradialion)'
f276dJo (20.1hja (IOO5dJo {55d)a {58",.,0: p.O monj 0
~~:;;~~~rl~
7.874
[Krj4d55s2 Technetium
(266)
-
-
¥©
11.5"
[Xe]4f'45d46s2 Tungsten
05~262)
(98) 7,5,4
43 4538 2430
{Kr]4d5Ss' Molybdenum 74 5828 3695
5730 3290 16.65
0
10.22
Nb
[Kr]4d4Ssl Niobium
n
5~;,~~?7
8
[Ar]3d54s2 Manganese
95.94 6,5,4,3,2
42
5017 2730
25 2234M 1519 7.44
7.19
4192.90;,~,~
1
[Rn)5ft46d27s2' Rutherfordiurn
V
7NIIA
6NIA
[Ar)3d34s2 Vanadium
~61)
-
5°·:~1:2
6.11
[Kr)4d2Ss2 Zirconium
a
L
91.224 4
40 4682 2128
[Xe]5d16s2 Lanthanum (226) 2
47.867 4,3,2
6.51
POINT, K
'
4/1VA
{Kr]4d15s2 Yttrium
* BOlu::MBE\R KE~Ej'GHTO;;~~T!~~1 ~:,;,~S POINT
(6L88hllr
[2M.ldiP+,E(
••w
(W.20d)/r (2.7)'!P-
in blue (although some are also rnanutactueeo). Letter m inan isomer 01 anomer isotope of the same mass number
Values
ATOMIC
MELTlN~
•."
4.54 Ti {Ar]3d24s2 Titanium
5c [Ar]3d'4s2 Scandium
57138.9~55
88 1413 973
Fr
• Estimated
2.99
[Xe]6s2 Barium
[Rn]7st Francium
(78.8d)P+,EC (270djEC (71.3d]p•.,EC 15·272Ylr (36.0h!P+,EC [8xlO'I1K (92YlP(12.70hlP-, p ". EC
22 3560 1941
4.47
5r
3.5
(223) 1
-
2144.95,591
3618 1795
2170 1000
[Xej6s1 Cesium 87 950' 300
3/11IA
56137}27
C
uN.
56 57 58 60 57
"""
[Kr)Ss2 Strontium
55132.9?545
(44.6d]{r
6)
3109 1814
2.54
.,Ge :uAs
67
65 67 72
124 125 121
number dicates
9
40.078 2
38 1655 1050
[Kr)Ssl Rubidium
944 301,54 1.87
9
Ca [Ar]4s2 Calcium
1.532
ss
,,<-
1.55
K
85.4~78
312.46 961
"
.Fe
24.;050
20 1757 1115
[Ar]4s' Potassium 37
,,1M
1363M 923 1.74 [Ne]3s2 Magnesium
1033 336.8 0.862
"c,
12
[Ne]3s' Sodium 19
,,"
Be [He]2s2 Beryllium
",In .,Go
"'"
.eo
1.85
a
1156.1N 371.0 0.971
9.012182 2
4 2744 1560
m
"
"Mg
t
P5_3 "ilr
3 1l2.26yl/r 7 (53.3diEC 10 1'.6~ IO'yl Ill' 12040m"ip+ 14 (5730Yllr 18 1l09_B~r 22 [2.602ylP,EC [I5.02,*P28 [20.9hItr 26 [7.2x IO'ylf3~,EC 32 II4.26d)p35 (87.2d) {r 36 13.01x IO'ylP36 (37.2m~p37 (35.Q2d]K 39 1265}'ltr (L28x 10'ylEC '0 112.36I1P(165d)pao 183.80d)P51 (27.70d)EC 53 (2xI0"I1EC (313.0d)EC 56 (2.578h1lr
,H
.,Sb
Naturally occurring
Side 1
TABLE OF PERIODIC PROPERTIES OF THE ELEMENTS Percent Ionic Character of a Single Chemical Bond Difference
GROUP
in electronegativity
0.1
0.20.3
0.4
0.5 0.6
0.7
0.8
0.9
1.0
1.1
1.2
1.3
1.4
1.5
1.6
1.7
1.8 1.92.0
2.1
2.2
2A
2.3
2.5
2.62.72.8
2.9
3.0
3.1
3.2
18/V1II
:L/IA Percentionicchoeccree 0.32 0.79 14.10 13.598 14.304
2.20 0.4581 0.0585 0.1815
%
DATA CONCERNING
0.5 1
2
6
9
12 '5
Neutron
Electron'
Proton
Symbol
e
P
Rest moss
1,67495)(10.27 1.67265x10·27
(kg)
Relativeotomiemoss ("C:12)
19 22 26 30 34 39 43 47 51 55 59 63 67 70 74 76 79 82 84 86 88 89 91 92
THE MORE STABLE ELEMENTARY (SUBATOMIC)
2/IIA
Charge
4
1.008665
(e)
Neutrino
9.1095xI0·31
~O
1.007276
5,48580xl 0.4
~o
1.60219x10·19
.1.60219)(10.19
1/2
1/2
-1,913JIN
Magnetic Momentt
CRYSTAL STRUCTURE
• The positron {e+] has properties similar to those of the (neg· ative) ele
photon
-0 1/2
Spin quantum number
2.793
0.93 0.49 31.80 24.587 5.193
PARTICLES
(e-)
Radius (m)
He@ 0.084 0.021 0.152
tJlB:Bohr mogneton ond J.IN=Nudeor magneton,
1/2
1.001 JIB
JiN
ACIO·BASE (2)
PROPERTIES
(I)
ELECTRONEGATIVITY,
(Pauling's)
SYMBOL
COVALENT
ATOMIC
RADIUS,
VA~~:I~~~ON kJ fmol (4)
A (7)
RADIUS, ATOMIC
HEAT
VOLUME,
OF FUSION
kJ/mol
cm'/mol{8)
FIRST
ELECTRICAL
IONIZATION
©Copyright
1992
15.0 5.89
o'n' m'
SPECIFIC HEAT CAPACITY, Jg" K' (3)
THERMAL
12.50 6.194 0.12
5.6 47
21.10 6.266
12.32 6.026 0.13'
0.7 6.74
valence)
of group.
Oxide
shown.
Intensity
0.8 6.3
20.8 5.974
18.3 5.991
0.7 10'
6.198
6.282
10'
10'
6.42 10'
6.5 10'
6.58 10'
6.65 10'
4.9'
to-
10'
(5)
(1) For representative and
(6)
(2)
@Copyrightl993
©Copyrighl1994 @Copyrighl1995 ©Copyright 1996 ©Copyright 1998 @Copyrighl2000 @CopyrighI2001 ©Copyrighl2002 @ Copyrighl 2004 e Copyrighl 2007
1.3 543.92 15.65 7.1 54
CONDUCTIVITY 1
V 1979 1980
19.90 6.31 0.113
NOTES:
POTENTIAL
e Copyright ©Copyright
**
A
CONDUCTIVITY, Wm·'K·'(3)
amphoteric
* ~
oxides if both
Cubic, face hexagonal;
(higher
colors
are
centered;
~
cubic,
@
rhombohedral;
(6)
Generally
body
LD
is acidic
e
af color indicates
centered;
UI
tetragonal;
if color
is red,
relative
basic
if color
is blue
strength. The A & B subgroup designations, are those recommended by the International Union of Pure and Applied Chemistry,
cubic;
orthorhombic;
0
manoclinic.
Sargent-Welch VWR'-J" INHRNAT10NAL
(3)
At 300 K (>7'C)
(4)
At boiling
(5)
At melting
point point
at 293
K (20°C)
for polycrystalline (7)
Quantum for frcc
mechanical
material value
(8)
From density and refer
at 300
solid clements: to liquid
state
K (27°C) for liquid values
for gaseous
at boiling
PO. Box 4130· Buffalo, NY 14217 1-800·727-4368· FAX 1-800-676-2540 www.sargenlwelch.com
elements
point
atom Catalog
Number
WLS·18806
SIDE
2
VVVR Intemalional
)
)
)