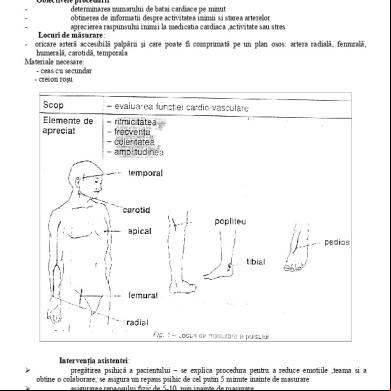
Me526 Lecture Note 1 Students 3t3470
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 2z6p3t
Overview 5o1f4z
& View Me526 Lecture Note 1 Students as PDF for free.
More details 6z3438
- Words: 4,017
- Pages: 22
ME 526 Fatigue and Fracture
ME 526 Fatigue and Fracture Analysis of Engineering Materials Dr. H.J. Kwon Assistant Professor, PEng University of Waterloo [email protected]
University of Waterloo
ME 526 Fatigue and Fracture
Contents 1. Introduction 2. Engineering Materials 3. Stress and Strain 4. Failure Criteria 5. Stress-Based Fatigue Analysis 6. Strain-Based Fatigue Analysis 7. Fracture Mechanics 8. Fatigue Crack Analysis
University of Waterloo
ME 526 Fatigue and Fracture
Chapter 1
1. Introduction 1.1 Economic Importance of Fracture
The study on the economic effects of fracture of materials in the United States by National Institute of Standards and Technology (formerly the National Bureau of Standards, 1983): - The total costs per year: $119 billion in 1982 dollars (4% of the gross national product) - Fracture includes cracking and deformation (wear and corrosion were not included). - Including wear and corrosion, the total cost increases up to 10% of the GNP.
In Europe and other industrial nations, the costs are around 4% of the GNP.
Costs of Fracture: $119 billion per year (1982) Could be eliminated through better use of current technology
Could perhaps be eliminated through research and development
Difficult to be eliminated without major breakthrough
The costs of fracture are spread over various sectors of the economy. - Vehicle and parts: 10% - Aircraft and parts: 6% - Residential construction: 5% - Building construction: 3% - Food and related products, fabricated structural products, nonferrous metal products, petroleum refining, structural metal, and tires and inner tubes: 2-3% - Health care related costs are rising.
University of Waterloo
1-1
ME 526 Fatigue and Fracture
Chapter 1
1.2 Types of material failure
Fig. 1.1 Basic types of deformation and fracture
1.3 Deformations and Material Behaviour Elastic and Plastic Deformation
University of Waterloo
1-2
ME 526 Fatigue and Fracture
Chapter 1
Elastic deformation
Plastic deformation
Fig. 1.2 Elastic and plastic deformation
Ductile and Brittle Behaviours
Fig. 1.3 Deformation behaviour
University of Waterloo
1-3
ME 526 Fatigue and Fracture
Chapter 1
Tension tests Tension tests are often employed to assess the strength and ductility of materials (Fig. 1.3). - Yield strength, 0 : the stress where plastic deformation begins. -
Ultimate tensile strength, u : the highest stress reached before fracture. Fracture strain, f : the strain at fracture, which is a measure of ductility and is usually expressed as a percentage, then being called the percent elongation. Toughness: the measure of material’s resistance to fracture, which refers to the energy needed to generate a unit of new crack surface. Materials having high values of both u and f are said to be tough, and tough materials are generally desirable for use in design.
Buckling Deformation due to compressive stress that causes large changes in alignment of columns or plates, perhaps to the extent of folding or collapses. Creep - Deformation that accumulates with time - Depending on the magnitude of the applied stress and its duration, the deformation may become so large that a component can no longer perform its function 1.4 Fracture Brittle Fracture - Accompanied by little plastic deformation - More likely to occur under impact loading - If a crack or other sharp flaw is present, brittle fracture can occur even in ductile materials that are normally fractured in a ductile manner - Resistance to brittle fracture in the presence of a crack is measured by fracture toughness, KIc - Materials with high strength generally have low fracture toughness, and vice versa.
Brittle fracture
Rapid crack propagation Ductile fracture
University of Waterloo
1-4
ME 526 Fatigue and Fracture
Chapter 1
1.5 Fatigue -
A failure due to repeated loading
High-cycle fatigue
Low-cycle fatigue
Fig. 1.4 Development of a fatigue crack.
University of Waterloo
1-5
ME 526 Fatigue and Fracture
Chapter 1
1.6 Safety Factors Safety Factors in Stress - The ratio of the stress that causes failure to the stress expected to occur in the actual service of the component.
(1.1) -
To avoid excessive deformation due to yielding, the failure stress is the yield strength, σ0, and the service stress is the largest stress in the component. Values for safety factors in the range X1 = 1.5 to 3.0 are common. If the magnitude of the loading is well known, values near the lower end of this range can be adopted.
Safety Factors in Life - The ratio of the expected life to failure to the desired service life. - Life is measured by time or by events such as the number of flights of an aircraft. (1.2) -
For example, if a helicopter part is expected to fail after 10 years of service, and if it is to be replaced after 2 years, X2 = 5. As the life is generally quite sensitive to small changes in stress, values of this factor must be relatively large, typically in the range X2 = 5 to 20.
Ex. 1.1 A plate as in Fig. 1.5 is subjected to a tension load. The tension load is P=14,400 N, and the dimensions are w=32, h=16, r=8 and t=5 mm. It is made of an aluminum alloy with yield strength σ0 =303 MPa. In a tension test, this material exhibits reasonably ductile behaviour, finally breaking at a strain around εf =20%. a) What is the safety factor against yielding? b) What is the safety factor against large amounts of deformation due to yielding?
University of Waterloo
1-6
ME 526 Fatigue and Fracture
Chapter 1
Fig. 1.5 Stress concentration factor
University of Waterloo
1-7
ME 526 Fatigue and Fracture
Chapter 2
2. Engineering Materials -
The materials that are used to resist to mechanical loading are called engineering materials. They can be broadly classified into four major classes: metals and alloys, polymers, ceramics and glasses, and composites. The difference of material properties are caused by chemical bonding and microstructure
Fig. 2.1 General characteristics of the major classes of engineering materials
2.1 Bonding Primary Bonds: ionic, covalent, metallic - Ionic bonding involves the transfer of one or more electrons between atoms; covalent bonding involves the sharing of electrons; a “cloud” of electrons are shared by the metal atoms in metallic bonding. - Strong and stiff, do not easily melt - Bonding of metals and ceramics
Fig. 2.2 The three types of primary chemical bond. Electrons are transferred in ionic bonding, as in NaCl; shared in covalent bonding, as in water; and given up to a common “cloud” in metallic bonding, as in magnesium metal.
University of Waterloo
2-1
ME 526 Fatigue and Fracture
Chapter 2
Secondary Bonds: Van der Waals, hydrogen - Secondary bonds occur due to the presence of an electrostatic dipole, which can be induced by a primary bond - Van der Waals bonds arise from the fluctuating positions of electrons relative to an atom’s nucleus; in water, the side of a hydrogen atom away from the covalent bond to the oxygen atom has a positive charge, while the exposed portion of the oxygen atom has a negative charge. The dipoles cause an attraction between adjacent molecules, that is called a hydrogen bond. - Relatively weak
(a)
(b)
Fig. 2.3 (a) Hydrogen-to-chlorine secondary bonds between chain molecule in polyvinyl chloride; (b) oxygen-to-hydrogen secondary bonds between water molecules.
2.2 Structures -
Metals and ceramics are composed of aggregations of small grains, each of which is an individual crystal. Glasses have an amorphous structure. Polymers are composed of chainlike molecules, which are sometimes arranged in regular arrays in a crystalline manner.
Crystal structure - The arrangement of atoms in crystals can be described in of the smallest grouping, called unit cell. - There are seven basic types of unit cell.
Fig. 2.4 The general case of a unit cell in a crystal and three of seven basic types.
University of Waterloo
2-2
ME 526 Fatigue and Fracture
Chapter 2
For a given type of unit cell, various arrangements of atoms are possible; each such arrangement is called a crystal structure. PC (primary cubic): rare BCC (body centered cubic): a number of common metals (chromium, iron, molybdenum, tungsten) FCC (face centered cubic): common metals (silver, aluminum, lead, copper, and nickel) H (hexagonal close-packed): beryllium, magnesium, titanium, and zinc
Fig. 2.5 Four crystal structures: PC (Primary Cubic), BCC (Body Centered Cubic), FCC (Face Centered cubic), H (Hexagonal close-packed) structures.
-
A material may change its crystal structure with temperature or pressure, or with the addition of alloying elements. E.g., the BCC structure of iron (-Fe) changes to FCC (-Fe) above 910C, and back to BCC (-Fe) above 1390C.
Structures in Polymers Amorphous: PMMA (polymethyl methacrylate), PS (polystyrene) and PC (polycarbonate). Semicrystalline: PE (polyethylene), PET(polyethylene terephthalate), PTFE (polytetrafluoroethylene), PP (polypropylene) Crystalline: thermosetting polymers
Fig. 2.6 Amorphous structure (left) and crystalline structure (right) in a polymer 2.3 Metals and Alloys -
Approximately 80% of the one-hundred-plus elements in the periodic table can be classed as metals. A metal alloy is usually a melted-together combination of two or more chemical elements, where the bulk of the material consists of one or more metals. University of Waterloo
2-3
ME 526 Fatigue and Fracture -
Chapter 2
Iron is the main constituent of the iron-based alloys (steels). A wide variety of metallic and nonmetallic chemical elements are used, e.g., boron, carbon, magnesium, silicon, vanadium, chromium, manganese, nickel, copper, zinc, molybdenum, and tin. The amounts and combinations of alloying elements used with various metals have major effects on their strength, ductility, temperature resistance, corrosion resistance, and other properties.
Table 2.1 Properties and uses for selected engineering metals and their alloys Metal
Melting Temp.
Tm °C (°F)
Density
Elastic Modulus
ρ E g/cm3 GPa (lb/ft3) (103 ksi)
Typical Strength
Uses; Comments
σu Mpa (ksi)
Iron (Fe) and steel
1538 (2800)
7.87 (491)
212 (30.7)
200 to 2500 (30 to 360)
Diverse: structures, machine and vehicle parts, tools. Most widely used engineering metal.
Aluminum (Al)
660 (1220)
2.70 (168)
70 (10.2)
140 to 550 (20 to 80)
Aircraft and other lightweight structure and parts.
Titanium (Ti)
1670 (3040)
4.51 (281)
120 (17.4)
340 to 1200 (50 to 170)
Aircraft structure and engines; industrial machine parts; surgical implants.
Copper (Cu)
1085 (1985)
8.93 (557)
130 (18.8)
170 to 1400 (25 to 200)
Electrical conductors; corrosion-resistant parts, valves, pipes. Alloyed to make bronze and brass.
Magnesium (Mg)
650 (1200)
1.74 (108)
45 (6.5)
170 to 340 (25 to 50)
Parts for high-speed machinery; aerospace parts.
Nickel (Ni)
1455 (2650)
8.90 (556)
210 (30.5)
340 to 1400 (50 to 200)
Jet engine parts; alloying addition for steels.
Cobalt (Co)
1495 (2720)
8.83 (551)
211 (30.6)
650 to 2000 (95 to 300)
Jet engine parts; wear resistant coatings; surgical implants.
Tungsten (W)
3422 (6190)
19.3 (1200)
411 (59.6)
120 to 650 (17 to 94)
Electrodes, light bulb filaments, flywheels, gyroscopes.
Lead (Pb)
328 (620)
11.3 (708)
16 (2.3)
12 to 80 (2 to 12)
Corrosion resistant piping; weights, shot. Alloyed with tin in solders.
Irons and Steels Iron-based alloys (ferrous alloys, e.g. cast irons and steels) and are the most widely used structural metals. Steels: iron + carbon + manganese + additional alloying elements. Pure iron: ingot iron, quite weak Cast irons: carbon in excess of 2% and from 1 to 3% silicon. * Pure iron is quite weak, but is strengthened considerably by the addition of small amounts of carbon. Additional alloying with small amounts of niobium, vanadium, copper, or other elements permits strengthening by grain refinement, precipitation, or solid solution effects. University of Waterloo
2-4
ME 526 Fatigue and Fracture
Chapter 2
* If sufficient carbon is added for quenching and tempering to be effective, a major increase in strength is possible. Table 2.2 Commonly encountered classes of iron and steels Class
Distinguishing Features
Typical Uses
Cast iron
More than 2% C and 1 to 3% Si
Pipes, valves, gears, engine blocks
Plain-carbon steel
Principal alloying element is carbon Structural and machine parts up to 1 %
Low-alloy steel
Metallic elements totaling up to 5% High-strength structural and machine parts
Stainless steel
At least 10% Cr; does not rust
Tool steel
Heat treatable to high hardness and Cutters, drill bits, dies wear resistance
Corrosion resistant piping and nuts and bolts; turbine blades
Table 2.3 Some typical irons and steels Description Ductile cast iron Low-carbon steel Medium-carbon steel High-carbon steel Low-alloy steel HSLA steel Martensitic stainless steel Austenitic stainless steel Precipitation hardening stainless steel Tungsten highspeed tool steel 18 Ni maraging steel
Identification ASTM A395 AISI 1020
Principal Alloying Elements, Typical % by Weight UNS No. C Cr Mn Mo Ni Si V Other F32800 3.5 2 G 10200 0.2 0.45 0.2 -
AISI 1045
G 10450 0.45 -
0.75 -
-
0.2
-
-
AISI 1095
G10950
0.95 -
0.4
-
-
0.2
-
-
AISI 4340 G43400 ASTM A588-A K11430 AISI 403 S40300
0.40 0.8 0.15 0.5 0.15 12
0.7 1.1 1.0
0.25 1.8 0.6
0.2 0.2 0.5
0.05 0.3 Cu -
AISI 310
S31000
0.25 25
2.0
-
20
1.5
-
-
17-4PH
SI 7400
0.07 17
1.0
-
4
1.0
-
4 Cu 0.3 Nb+Ta
AISI T1
T1200I
0.75 3.8
0.25 -
0.2
0.3
1.1
18 W
0.01 -
-
18
-
-
9 Co, 0.7 Ti
ASTM A538-C K93I20
University of Waterloo
5
2-5
ME 526 Fatigue and Fracture
Chapter 2
Naming systems for Irons and Steels American Iron and Steel Institute (AISI), the Society of Automotive Engineers (SAE), and the American Society for Testing and Materials (ASTM International) SAE and ASTM have cooperated to develop a new Unified Numbering System (UNS) that gives designations not only for irons and steels, but also for all other metal alloys. The AISI and SAE are nearly identical.
Table 2.4 Summary of the AISI-SAE Designation for Common Carbon and Low-Alloy Steels Designation
Approx. Alloy Content, %
Carbon steels
Designation
Approx. Alloy Content, %
Nickel-molybdenum steels
10XX
Plain carbon
46XX
Ni 0.85 or 1.82; Mo 0.25
11XX
Resulfurized
48XX
Ni 3.50; Mo 0.25
12XX
Resulfurized and rephosphorized
15XX
Mn 1.00 to 1.65
Manganese steels 13XX
Mn 1.75
Chromium steels 50XX(X) Cr 0.27 to 0.65 51XX(X)
Cr 0.80 to 1.05
52XXX
Cr 1.45
Molybdenum steels 40XX Mo 0.25 Mo 0.40 or 0.52 44XX
Chromium-vanadium steels 61XX Cr 0.6 to 0.95; V 0.15
Chromium-molybdenum steels
Silicon-manganese steels
41XX
92XX
Cr 0.50 to 0.95; Mo 0.12 to 0.30
Nickel-chromium-molybdenum steels 43XX Ni 1.82; Cr 0.50 or 0.80; Mo 0.25 47XX
Ni 1.45; Cr 0.45; Mo 0.20 or 0.35
81XX
Ni 0.30; Cr 0.40; Mo 0.12
86XX
Ni 0.55; CrO.50; Mo 0.20
87XX
Ni 0.55; Cr 0.50; Mo 0.25
94XX
Ni 0.45; Cr 0.40; Mo 0.12
University of Waterloo
Si 1.40 or 2.00; Mn 0.70 to 0.87; Cr 0 or 0.70
Boron steels YYBXX
B 0.0005 to 0.003
2-6
ME 526 Fatigue and Fracture -
Chapter 2
The UNS system has a letter followed by a five-digit number. The letter indicates the category of alloy (e.g., F for cast irons, G for carbon and low-alloy steels, K for various special-purpose steels, S for stainless steels, and T for tool steels). For carbon and low-alloy steels, the number is in most cases the same as that used by AISI and SAE, except that a zero is added at the end. Thus, AISI 1340 is the same steel as UNS G13400.
2.4 Polymers -
Consist of long-chain molecules formed primarily by carbon-to-carbon bonds Include plastics, natural and synthetic fibers, rubbers, and cellulose and lignin in wood Classified into three groups: thermoplastics, thermosetting plastics, and elastomers.
Table 2.5 Classes, Examples, and Uses of Representative Polymers Polymer (a) Thermoplastics: ethylene structure Polyethylene (PE) Polyvinyl chloride (PVC) Polypropylene (PP) Polystyrene (PS) Polymethyl methacrylate (PMMA, Plexiglas, acrylic) Polytetrafluoroethylene (PTFE, Teflon) Acrylonitrile butadiene styrene (ABS) (b) Thermoplastics: others Nylon Aramids (Kevlar, Nomex) Polyoxymethylene (POM, acetal) Polyetheretherketone (PEEK) Polycarbonate (PC) (c) Thermosetting plastics Phenol formaldehyde (phenolic, Bakelite) Melamine formaldehyde Urea formaldehyde Epoxies Unsaturated polyesters (d) Elastomers Natural rubber; cis-polyisoprene Styrene-butadiene rubber (SBR) Polyurethane elastomers Nitrile rubber Polychloroprene (Neoprene)
-
Typical Uses Packaging, bottles, piping Upholstery, tubing, electrical insulation Hinges, boxes, ropes Toys, appliance housings, foams Windows, lenses, clear shields, bone cement Tubing, bottles, seals Telephone and appliance housings, toys Gears, tire cords, tool housings High-strength fibers Gears, fan blades, pipe fittings Coatings, fans, impellers Safety helmets and lenses Electrical plugs and switches, pot handles Plastic dishes, tabletops Buttons, bottle caps, toilet seats Matrix for composites Fiberglass resin Shock absorbers, tires Tires, hoses, belts Shoe soles, electrical insulation O-rings, oil seals, hoses Wet suits, gaskets
Thermoplastics: soften and melt when heated; then, if cooled, it returns to its original solid condition. Thermosetting plastics do not melt upon reheating, but will instead decompose, as by charring or burning.
University of Waterloo
2-7
ME 526 Fatigue and Fracture -
-
Chapter 2
Elastomers are capable of rubbery behavior. They can be deformed by large amounts, say 100% to 200% strain or more, with most of this deformation being recovered after removal of the stress. Names are often abbreviated by acronyms, such as PMMA for polymethyl methacrylate. Various trade names and popular names, such as Plexiglas, Teflon, and nylon, are often used in addition to, or in place of, the chemical names. Light weight: around ρ = 1 g/cm3, and few exceed ρ = 2 g/cm3. (c.f. ρ = 2.7 for Al, ρ ~ 7.9 for steel). Most polymers are relatively weak, with ultimate tensile strengths typically in the range 10 to 200 MPa.
2.5 Ceramics -
-
Solids that are neither metallic nor organic (carbon-chain based) materials. Include clay products, such as porcelain, china, and brick, and also natural stone and concrete. Ceramics used in high-stress applications, called engineering ceramics, are often relatively simple compounds of metals, or the metalloids silicon or boron, with nonmetals such as oxygen, carbon, or nitrogen. Crystalline structure
Advantages Highly resistant to corrosion and wear, and melting temperatures are typically quite high. These characteristics all arise from the strong covalent or ionic-covalent chemical bonding of these compounds. Stiff (high E) and light in weight. Disadvantages Inherently brittle, because of covalent bonding The brittleness is further enhanced by the fact that grain boundaries in these crystalline compounds are relatively weaker than in metals. There are often an appreciable degree of porosity and microscopic cracks. These discontinuities promote macroscopic cracking and thus also contribute to brittle behavior. 2.6 Composite Materials -
Made by combining two or more materials that are mutually insoluble Examples: plastics modified by adding rubber particles, plastics reinforced by chopped glass fibers, cemented carbides, and concrete. Materials that are melted (alloyed) together are not considered composites Can be tailored to meet special needs such as high strength and stiffness combined with light weight. Increasingly being used in aircraft, space, and defense applications, and also for high-grade sports equipment, as in golf club shafts and fishing rods. Economical composites (e.g. glass-reinforced plastics) are used in a wide range of products, such as automotive components, boat hulls, sports equipment, and furniture
University of Waterloo
2-8
ME 526 Fatigue and Fracture
Chapter 2
Fig. 2.7 Composites reinforced by (a) particles, (b) chopped fibers, and (c) continuous fibers.
Table 2.6 Representative types and examples of composite materials Reinforcing Type
Matrix
Example
Typical Use
(a) Particulate composites Ductile polymer or elastomer Ceramic
Brittle polymer
Rubber in polystyrene, ABS
Toys, cameras
Ductile metal
Cutting tools
Ceramic
Ceramic
WC (Tungsten Carbide) with Co metal binder Granite, stone, and silica sand in Portland cement
Chopped glass in polyester resin
Auto body s
SiC whiskers in Al alloy
Aircraft structured s
Graphite in epoxy
Aircraft wing flaps
Boron in Al alloy SiC in Si3N4
Aircraft structure Engine parts
PVC and ABS sheets over ABS foam core Kevlar in epoxy between Al alloy layers (ARALL)
Canoes
Bridges, buildings
(b) Short-fiber, whisker composites Strong fiber Ceramic
Thermosetting plastic Ductile metal
(c) Continuous-fiber composites Ceramic Ceramic Ceramic
Thermosetting plastic Ductile metal Ceramic
(d) Laminated composites Stiff sheet
Foamed polymer
Composite
Metal
University of Waterloo
Aircraft structure
2-9
ME 526 Fatigue and Fracture
Chapter 2
2.7 Materials Selection -
-
An engineering component must not deform excessively or fail by fracture or collapse. At the same time, the cost and often the weight must not be excessive. To avoid excessive deformation, deflection due to elastic strain should be limited. For a given component geometry and applied load, the elastic modulus E of the material is the determining factor for elastic deformation. As to strength, the stress should not exceed the failure strength of the material, such as the yield strength σ0.
Selection Procedure 1. Classify the variables that enter the problem into categories as follows: - Requirements - Geometry that may vary - Material properties - Quantity to be minimized or maximized 2. Express the quantity Q to be minimized or maximized as a mathematical function of the requirements and the material properties, in which the geometry variable does not appear:
Q f1 (Requiremen ts) f 2 (Material)
(1.3)
3. Determine Q for each candidate material, and choose the one with smallest or largest value of Q, depending on the situation. Case Study: cantilever beam Consider the case of a cantilever beam having a circular cross section and a load at the end as in Fig. 2.8. The length of the beam is fixed, but the radius can be changed to minimize the weight.
Fig. 2.8 Cantilever beam with circular cross-section
University of Waterloo
2-10
ME 526 Fatigue and Fracture
Chapter 2
a. Understanding problem:
b. Variables
c. Mathematical expression of m:
University of Waterloo
2-11
ME 526 Fatigue and Fracture
Chapter 2
Ex. 2.1 For the beam in Fig. 2.8 and the materials of Table 2.7, proceed as follows: (a) Perform the materials selection for minimum mass. (b) Calculate the beam radius r that is required for each material. Assume values of P = 200N, L=100mm, and X=2. (c) Extend the analysis to a consideration of cost. Table 2.7 Selected Typical Materials Elastic Modulus E, GPa
Strength σc, MPa
Density 3 ρ,g/cm
Relative Cost, Cm
Material Type
Example
Structural (mild) steel
AISI 1020 steel
203
260
7.9
1
Low alloy steel
AISI 4340 steel
207
1103
7.9
3
High strength aluminum alloy
7075-T6 Al
71
469
2.7
6
Titanium alloy
Ti-6Al-4V
117
1185
4.5
45
Engineering polymer
Polycarbonate (PC)
2.4
62
1.2
5
Wood
Loblolly pine
12.3
88
0.51
1.5
Economical composite
Glass cloth in epoxy (GFRP)
21
380
2.0
10
High-performance composite
Graphite fiber in epoxy laminate (CFRP)
76
930
1.6
200
University of Waterloo
2-12
ME 526 Fatigue and Fracture
Chapter 2
Assignment #1 A leaf spring in the suspension system of an experimental vehicle is a beam of length L = 0.5m, with a rectangular cross section, as shown in Fig. A-1. This part, as currently designed with a low-alloy steel, has a width t = 60 mm and a depth h = 5 mm. However, if possible, it is desirable to replace this steel with another material to reduce the weight of the component. To avoid redeg other related parts, the t dimension should not be changed, but h can be varied, as long as it does not exceed 12 mm. The spring stiffness must be k = P/v = 50 kN/m. Also, the spring hits a limit to its motion at vmax = 30 mm, at which point the stress should not be so large that the safety factor against material failure is less than X = l.4. (a) Considering only the k = 50 kN/m requirement, determine which materials in Table 2.7 would provide a lighter weight component. (b) For each material, calculate the h necessary to meet the k = 50 kN/m requirement, and also the safety factor relative to c at vmax = 30 mm. Eliminate any materials that do not meet h≤12mm and X > 1.4. (c) Compare the alloy steel design with the use of each of the remaining candidates, considering cost and any other factors that you believe to be important.
Fig. A-1
Hint: - v max
PL3 P 48EI z th 3 , Iz → k 48EI z v 12 L3
- m thL where h can be expressed as the function of other variables.
University of Waterloo
2-13
ME 526 Fatigue and Fracture Analysis of Engineering Materials Dr. H.J. Kwon Assistant Professor, PEng University of Waterloo [email protected]
University of Waterloo
ME 526 Fatigue and Fracture
Contents 1. Introduction 2. Engineering Materials 3. Stress and Strain 4. Failure Criteria 5. Stress-Based Fatigue Analysis 6. Strain-Based Fatigue Analysis 7. Fracture Mechanics 8. Fatigue Crack Analysis
University of Waterloo
ME 526 Fatigue and Fracture
Chapter 1
1. Introduction 1.1 Economic Importance of Fracture
The study on the economic effects of fracture of materials in the United States by National Institute of Standards and Technology (formerly the National Bureau of Standards, 1983): - The total costs per year: $119 billion in 1982 dollars (4% of the gross national product) - Fracture includes cracking and deformation (wear and corrosion were not included). - Including wear and corrosion, the total cost increases up to 10% of the GNP.
In Europe and other industrial nations, the costs are around 4% of the GNP.
Costs of Fracture: $119 billion per year (1982) Could be eliminated through better use of current technology
Could perhaps be eliminated through research and development
Difficult to be eliminated without major breakthrough
The costs of fracture are spread over various sectors of the economy. - Vehicle and parts: 10% - Aircraft and parts: 6% - Residential construction: 5% - Building construction: 3% - Food and related products, fabricated structural products, nonferrous metal products, petroleum refining, structural metal, and tires and inner tubes: 2-3% - Health care related costs are rising.
University of Waterloo
1-1
ME 526 Fatigue and Fracture
Chapter 1
1.2 Types of material failure
Fig. 1.1 Basic types of deformation and fracture
1.3 Deformations and Material Behaviour Elastic and Plastic Deformation
University of Waterloo
1-2
ME 526 Fatigue and Fracture
Chapter 1
Elastic deformation
Plastic deformation
Fig. 1.2 Elastic and plastic deformation
Ductile and Brittle Behaviours
Fig. 1.3 Deformation behaviour
University of Waterloo
1-3
ME 526 Fatigue and Fracture
Chapter 1
Tension tests Tension tests are often employed to assess the strength and ductility of materials (Fig. 1.3). - Yield strength, 0 : the stress where plastic deformation begins. -
Ultimate tensile strength, u : the highest stress reached before fracture. Fracture strain, f : the strain at fracture, which is a measure of ductility and is usually expressed as a percentage, then being called the percent elongation. Toughness: the measure of material’s resistance to fracture, which refers to the energy needed to generate a unit of new crack surface. Materials having high values of both u and f are said to be tough, and tough materials are generally desirable for use in design.
Buckling Deformation due to compressive stress that causes large changes in alignment of columns or plates, perhaps to the extent of folding or collapses. Creep - Deformation that accumulates with time - Depending on the magnitude of the applied stress and its duration, the deformation may become so large that a component can no longer perform its function 1.4 Fracture Brittle Fracture - Accompanied by little plastic deformation - More likely to occur under impact loading - If a crack or other sharp flaw is present, brittle fracture can occur even in ductile materials that are normally fractured in a ductile manner - Resistance to brittle fracture in the presence of a crack is measured by fracture toughness, KIc - Materials with high strength generally have low fracture toughness, and vice versa.
Brittle fracture
Rapid crack propagation Ductile fracture
University of Waterloo
1-4
ME 526 Fatigue and Fracture
Chapter 1
1.5 Fatigue -
A failure due to repeated loading
High-cycle fatigue
Low-cycle fatigue
Fig. 1.4 Development of a fatigue crack.
University of Waterloo
1-5
ME 526 Fatigue and Fracture
Chapter 1
1.6 Safety Factors Safety Factors in Stress - The ratio of the stress that causes failure to the stress expected to occur in the actual service of the component.
(1.1) -
To avoid excessive deformation due to yielding, the failure stress is the yield strength, σ0, and the service stress is the largest stress in the component. Values for safety factors in the range X1 = 1.5 to 3.0 are common. If the magnitude of the loading is well known, values near the lower end of this range can be adopted.
Safety Factors in Life - The ratio of the expected life to failure to the desired service life. - Life is measured by time or by events such as the number of flights of an aircraft. (1.2) -
For example, if a helicopter part is expected to fail after 10 years of service, and if it is to be replaced after 2 years, X2 = 5. As the life is generally quite sensitive to small changes in stress, values of this factor must be relatively large, typically in the range X2 = 5 to 20.
Ex. 1.1 A plate as in Fig. 1.5 is subjected to a tension load. The tension load is P=14,400 N, and the dimensions are w=32, h=16, r=8 and t=5 mm. It is made of an aluminum alloy with yield strength σ0 =303 MPa. In a tension test, this material exhibits reasonably ductile behaviour, finally breaking at a strain around εf =20%. a) What is the safety factor against yielding? b) What is the safety factor against large amounts of deformation due to yielding?
University of Waterloo
1-6
ME 526 Fatigue and Fracture
Chapter 1
Fig. 1.5 Stress concentration factor
University of Waterloo
1-7
ME 526 Fatigue and Fracture
Chapter 2
2. Engineering Materials -
The materials that are used to resist to mechanical loading are called engineering materials. They can be broadly classified into four major classes: metals and alloys, polymers, ceramics and glasses, and composites. The difference of material properties are caused by chemical bonding and microstructure
Fig. 2.1 General characteristics of the major classes of engineering materials
2.1 Bonding Primary Bonds: ionic, covalent, metallic - Ionic bonding involves the transfer of one or more electrons between atoms; covalent bonding involves the sharing of electrons; a “cloud” of electrons are shared by the metal atoms in metallic bonding. - Strong and stiff, do not easily melt - Bonding of metals and ceramics
Fig. 2.2 The three types of primary chemical bond. Electrons are transferred in ionic bonding, as in NaCl; shared in covalent bonding, as in water; and given up to a common “cloud” in metallic bonding, as in magnesium metal.
University of Waterloo
2-1
ME 526 Fatigue and Fracture
Chapter 2
Secondary Bonds: Van der Waals, hydrogen - Secondary bonds occur due to the presence of an electrostatic dipole, which can be induced by a primary bond - Van der Waals bonds arise from the fluctuating positions of electrons relative to an atom’s nucleus; in water, the side of a hydrogen atom away from the covalent bond to the oxygen atom has a positive charge, while the exposed portion of the oxygen atom has a negative charge. The dipoles cause an attraction between adjacent molecules, that is called a hydrogen bond. - Relatively weak
(a)
(b)
Fig. 2.3 (a) Hydrogen-to-chlorine secondary bonds between chain molecule in polyvinyl chloride; (b) oxygen-to-hydrogen secondary bonds between water molecules.
2.2 Structures -
Metals and ceramics are composed of aggregations of small grains, each of which is an individual crystal. Glasses have an amorphous structure. Polymers are composed of chainlike molecules, which are sometimes arranged in regular arrays in a crystalline manner.
Crystal structure - The arrangement of atoms in crystals can be described in of the smallest grouping, called unit cell. - There are seven basic types of unit cell.
Fig. 2.4 The general case of a unit cell in a crystal and three of seven basic types.
University of Waterloo
2-2
ME 526 Fatigue and Fracture
Chapter 2
For a given type of unit cell, various arrangements of atoms are possible; each such arrangement is called a crystal structure. PC (primary cubic): rare BCC (body centered cubic): a number of common metals (chromium, iron, molybdenum, tungsten) FCC (face centered cubic): common metals (silver, aluminum, lead, copper, and nickel) H (hexagonal close-packed): beryllium, magnesium, titanium, and zinc
Fig. 2.5 Four crystal structures: PC (Primary Cubic), BCC (Body Centered Cubic), FCC (Face Centered cubic), H (Hexagonal close-packed) structures.
-
A material may change its crystal structure with temperature or pressure, or with the addition of alloying elements. E.g., the BCC structure of iron (-Fe) changes to FCC (-Fe) above 910C, and back to BCC (-Fe) above 1390C.
Structures in Polymers Amorphous: PMMA (polymethyl methacrylate), PS (polystyrene) and PC (polycarbonate). Semicrystalline: PE (polyethylene), PET(polyethylene terephthalate), PTFE (polytetrafluoroethylene), PP (polypropylene) Crystalline: thermosetting polymers
Fig. 2.6 Amorphous structure (left) and crystalline structure (right) in a polymer 2.3 Metals and Alloys -
Approximately 80% of the one-hundred-plus elements in the periodic table can be classed as metals. A metal alloy is usually a melted-together combination of two or more chemical elements, where the bulk of the material consists of one or more metals. University of Waterloo
2-3
ME 526 Fatigue and Fracture -
Chapter 2
Iron is the main constituent of the iron-based alloys (steels). A wide variety of metallic and nonmetallic chemical elements are used, e.g., boron, carbon, magnesium, silicon, vanadium, chromium, manganese, nickel, copper, zinc, molybdenum, and tin. The amounts and combinations of alloying elements used with various metals have major effects on their strength, ductility, temperature resistance, corrosion resistance, and other properties.
Table 2.1 Properties and uses for selected engineering metals and their alloys Metal
Melting Temp.
Tm °C (°F)
Density
Elastic Modulus
ρ E g/cm3 GPa (lb/ft3) (103 ksi)
Typical Strength
Uses; Comments
σu Mpa (ksi)
Iron (Fe) and steel
1538 (2800)
7.87 (491)
212 (30.7)
200 to 2500 (30 to 360)
Diverse: structures, machine and vehicle parts, tools. Most widely used engineering metal.
Aluminum (Al)
660 (1220)
2.70 (168)
70 (10.2)
140 to 550 (20 to 80)
Aircraft and other lightweight structure and parts.
Titanium (Ti)
1670 (3040)
4.51 (281)
120 (17.4)
340 to 1200 (50 to 170)
Aircraft structure and engines; industrial machine parts; surgical implants.
Copper (Cu)
1085 (1985)
8.93 (557)
130 (18.8)
170 to 1400 (25 to 200)
Electrical conductors; corrosion-resistant parts, valves, pipes. Alloyed to make bronze and brass.
Magnesium (Mg)
650 (1200)
1.74 (108)
45 (6.5)
170 to 340 (25 to 50)
Parts for high-speed machinery; aerospace parts.
Nickel (Ni)
1455 (2650)
8.90 (556)
210 (30.5)
340 to 1400 (50 to 200)
Jet engine parts; alloying addition for steels.
Cobalt (Co)
1495 (2720)
8.83 (551)
211 (30.6)
650 to 2000 (95 to 300)
Jet engine parts; wear resistant coatings; surgical implants.
Tungsten (W)
3422 (6190)
19.3 (1200)
411 (59.6)
120 to 650 (17 to 94)
Electrodes, light bulb filaments, flywheels, gyroscopes.
Lead (Pb)
328 (620)
11.3 (708)
16 (2.3)
12 to 80 (2 to 12)
Corrosion resistant piping; weights, shot. Alloyed with tin in solders.
Irons and Steels Iron-based alloys (ferrous alloys, e.g. cast irons and steels) and are the most widely used structural metals. Steels: iron + carbon + manganese + additional alloying elements. Pure iron: ingot iron, quite weak Cast irons: carbon in excess of 2% and from 1 to 3% silicon. * Pure iron is quite weak, but is strengthened considerably by the addition of small amounts of carbon. Additional alloying with small amounts of niobium, vanadium, copper, or other elements permits strengthening by grain refinement, precipitation, or solid solution effects. University of Waterloo
2-4
ME 526 Fatigue and Fracture
Chapter 2
* If sufficient carbon is added for quenching and tempering to be effective, a major increase in strength is possible. Table 2.2 Commonly encountered classes of iron and steels Class
Distinguishing Features
Typical Uses
Cast iron
More than 2% C and 1 to 3% Si
Pipes, valves, gears, engine blocks
Plain-carbon steel
Principal alloying element is carbon Structural and machine parts up to 1 %
Low-alloy steel
Metallic elements totaling up to 5% High-strength structural and machine parts
Stainless steel
At least 10% Cr; does not rust
Tool steel
Heat treatable to high hardness and Cutters, drill bits, dies wear resistance
Corrosion resistant piping and nuts and bolts; turbine blades
Table 2.3 Some typical irons and steels Description Ductile cast iron Low-carbon steel Medium-carbon steel High-carbon steel Low-alloy steel HSLA steel Martensitic stainless steel Austenitic stainless steel Precipitation hardening stainless steel Tungsten highspeed tool steel 18 Ni maraging steel
Identification ASTM A395 AISI 1020
Principal Alloying Elements, Typical % by Weight UNS No. C Cr Mn Mo Ni Si V Other F32800 3.5 2 G 10200 0.2 0.45 0.2 -
AISI 1045
G 10450 0.45 -
0.75 -
-
0.2
-
-
AISI 1095
G10950
0.95 -
0.4
-
-
0.2
-
-
AISI 4340 G43400 ASTM A588-A K11430 AISI 403 S40300
0.40 0.8 0.15 0.5 0.15 12
0.7 1.1 1.0
0.25 1.8 0.6
0.2 0.2 0.5
0.05 0.3 Cu -
AISI 310
S31000
0.25 25
2.0
-
20
1.5
-
-
17-4PH
SI 7400
0.07 17
1.0
-
4
1.0
-
4 Cu 0.3 Nb+Ta
AISI T1
T1200I
0.75 3.8
0.25 -
0.2
0.3
1.1
18 W
0.01 -
-
18
-
-
9 Co, 0.7 Ti
ASTM A538-C K93I20
University of Waterloo
5
2-5
ME 526 Fatigue and Fracture
Chapter 2
Naming systems for Irons and Steels American Iron and Steel Institute (AISI), the Society of Automotive Engineers (SAE), and the American Society for Testing and Materials (ASTM International) SAE and ASTM have cooperated to develop a new Unified Numbering System (UNS) that gives designations not only for irons and steels, but also for all other metal alloys. The AISI and SAE are nearly identical.
Table 2.4 Summary of the AISI-SAE Designation for Common Carbon and Low-Alloy Steels Designation
Approx. Alloy Content, %
Carbon steels
Designation
Approx. Alloy Content, %
Nickel-molybdenum steels
10XX
Plain carbon
46XX
Ni 0.85 or 1.82; Mo 0.25
11XX
Resulfurized
48XX
Ni 3.50; Mo 0.25
12XX
Resulfurized and rephosphorized
15XX
Mn 1.00 to 1.65
Manganese steels 13XX
Mn 1.75
Chromium steels 50XX(X) Cr 0.27 to 0.65 51XX(X)
Cr 0.80 to 1.05
52XXX
Cr 1.45
Molybdenum steels 40XX Mo 0.25 Mo 0.40 or 0.52 44XX
Chromium-vanadium steels 61XX Cr 0.6 to 0.95; V 0.15
Chromium-molybdenum steels
Silicon-manganese steels
41XX
92XX
Cr 0.50 to 0.95; Mo 0.12 to 0.30
Nickel-chromium-molybdenum steels 43XX Ni 1.82; Cr 0.50 or 0.80; Mo 0.25 47XX
Ni 1.45; Cr 0.45; Mo 0.20 or 0.35
81XX
Ni 0.30; Cr 0.40; Mo 0.12
86XX
Ni 0.55; CrO.50; Mo 0.20
87XX
Ni 0.55; Cr 0.50; Mo 0.25
94XX
Ni 0.45; Cr 0.40; Mo 0.12
University of Waterloo
Si 1.40 or 2.00; Mn 0.70 to 0.87; Cr 0 or 0.70
Boron steels YYBXX
B 0.0005 to 0.003
2-6
ME 526 Fatigue and Fracture -
Chapter 2
The UNS system has a letter followed by a five-digit number. The letter indicates the category of alloy (e.g., F for cast irons, G for carbon and low-alloy steels, K for various special-purpose steels, S for stainless steels, and T for tool steels). For carbon and low-alloy steels, the number is in most cases the same as that used by AISI and SAE, except that a zero is added at the end. Thus, AISI 1340 is the same steel as UNS G13400.
2.4 Polymers -
Consist of long-chain molecules formed primarily by carbon-to-carbon bonds Include plastics, natural and synthetic fibers, rubbers, and cellulose and lignin in wood Classified into three groups: thermoplastics, thermosetting plastics, and elastomers.
Table 2.5 Classes, Examples, and Uses of Representative Polymers Polymer (a) Thermoplastics: ethylene structure Polyethylene (PE) Polyvinyl chloride (PVC) Polypropylene (PP) Polystyrene (PS) Polymethyl methacrylate (PMMA, Plexiglas, acrylic) Polytetrafluoroethylene (PTFE, Teflon) Acrylonitrile butadiene styrene (ABS) (b) Thermoplastics: others Nylon Aramids (Kevlar, Nomex) Polyoxymethylene (POM, acetal) Polyetheretherketone (PEEK) Polycarbonate (PC) (c) Thermosetting plastics Phenol formaldehyde (phenolic, Bakelite) Melamine formaldehyde Urea formaldehyde Epoxies Unsaturated polyesters (d) Elastomers Natural rubber; cis-polyisoprene Styrene-butadiene rubber (SBR) Polyurethane elastomers Nitrile rubber Polychloroprene (Neoprene)
-
Typical Uses Packaging, bottles, piping Upholstery, tubing, electrical insulation Hinges, boxes, ropes Toys, appliance housings, foams Windows, lenses, clear shields, bone cement Tubing, bottles, seals Telephone and appliance housings, toys Gears, tire cords, tool housings High-strength fibers Gears, fan blades, pipe fittings Coatings, fans, impellers Safety helmets and lenses Electrical plugs and switches, pot handles Plastic dishes, tabletops Buttons, bottle caps, toilet seats Matrix for composites Fiberglass resin Shock absorbers, tires Tires, hoses, belts Shoe soles, electrical insulation O-rings, oil seals, hoses Wet suits, gaskets
Thermoplastics: soften and melt when heated; then, if cooled, it returns to its original solid condition. Thermosetting plastics do not melt upon reheating, but will instead decompose, as by charring or burning.
University of Waterloo
2-7
ME 526 Fatigue and Fracture -
-
Chapter 2
Elastomers are capable of rubbery behavior. They can be deformed by large amounts, say 100% to 200% strain or more, with most of this deformation being recovered after removal of the stress. Names are often abbreviated by acronyms, such as PMMA for polymethyl methacrylate. Various trade names and popular names, such as Plexiglas, Teflon, and nylon, are often used in addition to, or in place of, the chemical names. Light weight: around ρ = 1 g/cm3, and few exceed ρ = 2 g/cm3. (c.f. ρ = 2.7 for Al, ρ ~ 7.9 for steel). Most polymers are relatively weak, with ultimate tensile strengths typically in the range 10 to 200 MPa.
2.5 Ceramics -
-
Solids that are neither metallic nor organic (carbon-chain based) materials. Include clay products, such as porcelain, china, and brick, and also natural stone and concrete. Ceramics used in high-stress applications, called engineering ceramics, are often relatively simple compounds of metals, or the metalloids silicon or boron, with nonmetals such as oxygen, carbon, or nitrogen. Crystalline structure
Advantages Highly resistant to corrosion and wear, and melting temperatures are typically quite high. These characteristics all arise from the strong covalent or ionic-covalent chemical bonding of these compounds. Stiff (high E) and light in weight. Disadvantages Inherently brittle, because of covalent bonding The brittleness is further enhanced by the fact that grain boundaries in these crystalline compounds are relatively weaker than in metals. There are often an appreciable degree of porosity and microscopic cracks. These discontinuities promote macroscopic cracking and thus also contribute to brittle behavior. 2.6 Composite Materials -
Made by combining two or more materials that are mutually insoluble Examples: plastics modified by adding rubber particles, plastics reinforced by chopped glass fibers, cemented carbides, and concrete. Materials that are melted (alloyed) together are not considered composites Can be tailored to meet special needs such as high strength and stiffness combined with light weight. Increasingly being used in aircraft, space, and defense applications, and also for high-grade sports equipment, as in golf club shafts and fishing rods. Economical composites (e.g. glass-reinforced plastics) are used in a wide range of products, such as automotive components, boat hulls, sports equipment, and furniture
University of Waterloo
2-8
ME 526 Fatigue and Fracture
Chapter 2
Fig. 2.7 Composites reinforced by (a) particles, (b) chopped fibers, and (c) continuous fibers.
Table 2.6 Representative types and examples of composite materials Reinforcing Type
Matrix
Example
Typical Use
(a) Particulate composites Ductile polymer or elastomer Ceramic
Brittle polymer
Rubber in polystyrene, ABS
Toys, cameras
Ductile metal
Cutting tools
Ceramic
Ceramic
WC (Tungsten Carbide) with Co metal binder Granite, stone, and silica sand in Portland cement
Chopped glass in polyester resin
Auto body s
SiC whiskers in Al alloy
Aircraft structured s
Graphite in epoxy
Aircraft wing flaps
Boron in Al alloy SiC in Si3N4
Aircraft structure Engine parts
PVC and ABS sheets over ABS foam core Kevlar in epoxy between Al alloy layers (ARALL)
Canoes
Bridges, buildings
(b) Short-fiber, whisker composites Strong fiber Ceramic
Thermosetting plastic Ductile metal
(c) Continuous-fiber composites Ceramic Ceramic Ceramic
Thermosetting plastic Ductile metal Ceramic
(d) Laminated composites Stiff sheet
Foamed polymer
Composite
Metal
University of Waterloo
Aircraft structure
2-9
ME 526 Fatigue and Fracture
Chapter 2
2.7 Materials Selection -
-
An engineering component must not deform excessively or fail by fracture or collapse. At the same time, the cost and often the weight must not be excessive. To avoid excessive deformation, deflection due to elastic strain should be limited. For a given component geometry and applied load, the elastic modulus E of the material is the determining factor for elastic deformation. As to strength, the stress should not exceed the failure strength of the material, such as the yield strength σ0.
Selection Procedure 1. Classify the variables that enter the problem into categories as follows: - Requirements - Geometry that may vary - Material properties - Quantity to be minimized or maximized 2. Express the quantity Q to be minimized or maximized as a mathematical function of the requirements and the material properties, in which the geometry variable does not appear:
Q f1 (Requiremen ts) f 2 (Material)
(1.3)
3. Determine Q for each candidate material, and choose the one with smallest or largest value of Q, depending on the situation. Case Study: cantilever beam Consider the case of a cantilever beam having a circular cross section and a load at the end as in Fig. 2.8. The length of the beam is fixed, but the radius can be changed to minimize the weight.
Fig. 2.8 Cantilever beam with circular cross-section
University of Waterloo
2-10
ME 526 Fatigue and Fracture
Chapter 2
a. Understanding problem:
b. Variables
c. Mathematical expression of m:
University of Waterloo
2-11
ME 526 Fatigue and Fracture
Chapter 2
Ex. 2.1 For the beam in Fig. 2.8 and the materials of Table 2.7, proceed as follows: (a) Perform the materials selection for minimum mass. (b) Calculate the beam radius r that is required for each material. Assume values of P = 200N, L=100mm, and X=2. (c) Extend the analysis to a consideration of cost. Table 2.7 Selected Typical Materials Elastic Modulus E, GPa
Strength σc, MPa
Density 3 ρ,g/cm
Relative Cost, Cm
Material Type
Example
Structural (mild) steel
AISI 1020 steel
203
260
7.9
1
Low alloy steel
AISI 4340 steel
207
1103
7.9
3
High strength aluminum alloy
7075-T6 Al
71
469
2.7
6
Titanium alloy
Ti-6Al-4V
117
1185
4.5
45
Engineering polymer
Polycarbonate (PC)
2.4
62
1.2
5
Wood
Loblolly pine
12.3
88
0.51
1.5
Economical composite
Glass cloth in epoxy (GFRP)
21
380
2.0
10
High-performance composite
Graphite fiber in epoxy laminate (CFRP)
76
930
1.6
200
University of Waterloo
2-12
ME 526 Fatigue and Fracture
Chapter 2
Assignment #1 A leaf spring in the suspension system of an experimental vehicle is a beam of length L = 0.5m, with a rectangular cross section, as shown in Fig. A-1. This part, as currently designed with a low-alloy steel, has a width t = 60 mm and a depth h = 5 mm. However, if possible, it is desirable to replace this steel with another material to reduce the weight of the component. To avoid redeg other related parts, the t dimension should not be changed, but h can be varied, as long as it does not exceed 12 mm. The spring stiffness must be k = P/v = 50 kN/m. Also, the spring hits a limit to its motion at vmax = 30 mm, at which point the stress should not be so large that the safety factor against material failure is less than X = l.4. (a) Considering only the k = 50 kN/m requirement, determine which materials in Table 2.7 would provide a lighter weight component. (b) For each material, calculate the h necessary to meet the k = 50 kN/m requirement, and also the safety factor relative to c at vmax = 30 mm. Eliminate any materials that do not meet h≤12mm and X > 1.4. (c) Compare the alloy steel design with the use of each of the remaining candidates, considering cost and any other factors that you believe to be important.
Fig. A-1
Hint: - v max
PL3 P 48EI z th 3 , Iz → k 48EI z v 12 L3
- m thL where h can be expressed as the function of other variables.
University of Waterloo
2-13