General Chemistry For Grade 11 Course Outline 6s704k
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 2z6p3t
Overview 5o1f4z
& View General Chemistry For Grade 11 Course Outline as PDF for free.
More details 6z3438
- Words: 442
- Pages: 3
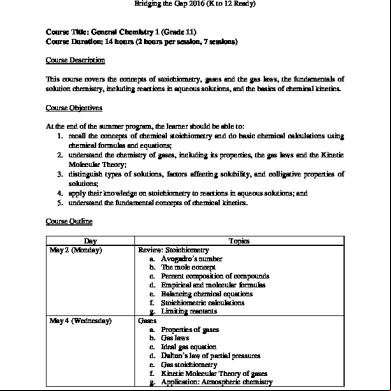
UNIVERSITY OF SAN CARLOS–PATHWAYS Bridging the Gap 2016 (K to 12 Ready)
Course Title: General Chemistry 1 (Grade 11) Course Duration: 14 hours (2 hours per session, 7 sessions) Course Description This course covers the concepts of stoichiometry, gases and the gas laws, the fundamentals of solution chemistry, including reactions in aqueous solutions, and the basics of chemical kinetics. Course Objectives At the end of the summer program, the learner should be able to: 1. recall the concepts of chemical stoichiometry and do basic chemical calculations using chemical formulas and equations; 2. understand the chemistry of gases, including its properties, the gas laws and the Kinetic Molecular Theory; 3. distinguish types of solutions, factors affecting solubility, and colligative properties of solutions; 4. apply their knowledge on stoichiometry to reactions in aqueous solutions; and 5. understand the fundamental concepts of chemical kinetics. Course Outline Day May 2 (Monday)
May 4 (Wednesday)
Topics Review: Stoichiometry a. Avogadro’s number b. The mole concept c. Percent composition of compounds d. Empirical and molecular formulas e. Balancing chemical equations f. Stoichiometric calculations g. Limiting reactants Gases a. Properties of gases b. Gas laws c. Ideal gas equation d. Dalton’s law of partial pressures e. Gas stoichiometry f. Kinetic Molecular Theory of gases g. Application: Atmospheric chemistry
May 9 (Monday)
May 11 (Wednesday) May 16 (Monday)
May 18 (Wednesday) May 23 (Monday)
May 25 (Wednesday)
Alternative Learning Activity: Physical Properties of Solutions (to be given as a module) a. Solutions and their composition b. Types of solutions c. Factors affecting solubility d. Methods of expressing concentration of solutions e. Colligative properties of solutions 1. Physical Properties of Solutions (Discussion) 2. Reactions in Aqueous Solutions a. Composition of solutions b. Types of solution reactions c. Precipitation reactions d. Acid–base reactions e. Reduction–oxidation reactions Chemical Kinetics Reaction rates Factors affecting reaction rates Rate laws Order of reactions Rate constant Application: Enzymes FINAL EXAM
Topics and activities may change without prior notice. Course Requirements
Modules (both in–class and take–home) Reading assignments Group reports Homework assignments and problem sets Working email address Final exam (may be waived in place of a take–home reflective essay)
Grading System
Performance tasks = 50% o Modules o Oral participation Written work = 25% o Problem sets o Quizzes Final examination = 25%
Teacher Information Name: Michael Ceniza Guinita Email: [email protected] Mobile Phone Number: +63 922 219 2085 Consultation Schedule Teacher is available for consultation upon request. Each student must inform the teacher if he/she needs a consultation session ahead of time. Both parties must agree on the schedule of the session.
Course Title: General Chemistry 1 (Grade 11) Course Duration: 14 hours (2 hours per session, 7 sessions) Course Description This course covers the concepts of stoichiometry, gases and the gas laws, the fundamentals of solution chemistry, including reactions in aqueous solutions, and the basics of chemical kinetics. Course Objectives At the end of the summer program, the learner should be able to: 1. recall the concepts of chemical stoichiometry and do basic chemical calculations using chemical formulas and equations; 2. understand the chemistry of gases, including its properties, the gas laws and the Kinetic Molecular Theory; 3. distinguish types of solutions, factors affecting solubility, and colligative properties of solutions; 4. apply their knowledge on stoichiometry to reactions in aqueous solutions; and 5. understand the fundamental concepts of chemical kinetics. Course Outline Day May 2 (Monday)
May 4 (Wednesday)
Topics Review: Stoichiometry a. Avogadro’s number b. The mole concept c. Percent composition of compounds d. Empirical and molecular formulas e. Balancing chemical equations f. Stoichiometric calculations g. Limiting reactants Gases a. Properties of gases b. Gas laws c. Ideal gas equation d. Dalton’s law of partial pressures e. Gas stoichiometry f. Kinetic Molecular Theory of gases g. Application: Atmospheric chemistry
May 9 (Monday)
May 11 (Wednesday) May 16 (Monday)
May 18 (Wednesday) May 23 (Monday)
May 25 (Wednesday)
Alternative Learning Activity: Physical Properties of Solutions (to be given as a module) a. Solutions and their composition b. Types of solutions c. Factors affecting solubility d. Methods of expressing concentration of solutions e. Colligative properties of solutions 1. Physical Properties of Solutions (Discussion) 2. Reactions in Aqueous Solutions a. Composition of solutions b. Types of solution reactions c. Precipitation reactions d. Acid–base reactions e. Reduction–oxidation reactions Chemical Kinetics Reaction rates Factors affecting reaction rates Rate laws Order of reactions Rate constant Application: Enzymes FINAL EXAM
Topics and activities may change without prior notice. Course Requirements
Modules (both in–class and take–home) Reading assignments Group reports Homework assignments and problem sets Working email address Final exam (may be waived in place of a take–home reflective essay)
Grading System
Performance tasks = 50% o Modules o Oral participation Written work = 25% o Problem sets o Quizzes Final examination = 25%
Teacher Information Name: Michael Ceniza Guinita Email: [email protected] Mobile Phone Number: +63 922 219 2085 Consultation Schedule Teacher is available for consultation upon request. Each student must inform the teacher if he/she needs a consultation session ahead of time. Both parties must agree on the schedule of the session.