Experiment 1 Coagulation And Flocculation i2f5e
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 2z6p3t
Overview 5o1f4z
& View Experiment 1 Coagulation And Flocculation as PDF for free.
More details 6z3438
- Words: 520
- Pages: 6
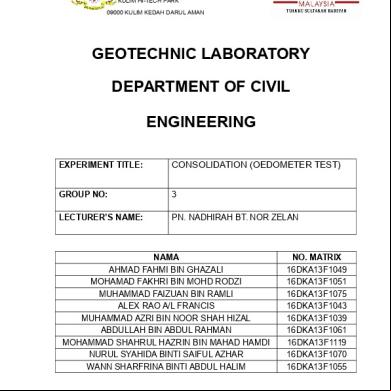
ENVIRONMENTAL ENGINEERING LABORATORY EXPERIMENT 1 : COAGULATION AND FLOACCULATION
1.1
i. ii. iii.
1.2
Objective
To determine the optimum alum dose pH for suspended solids removal from water using jar test. To determine the optimum concentration of coagulant to be added to the source water.
Coagulation and Flocculation
Suspended solids (particularly colloids) are often removed from water by chemical coagulation and flocculation. Colloidal particles carry a small electrostatic charge that keeps them in suspension. Coagulation is the addition of chemicals to neutralize the effect of colloidal charges and allow aggregation of particles. Following coagulation the suspension is stirred gently to promote particle collision and agglomeration in a process termed flocculation.
The type of source water will have a large impact on how often jar tests are performed. Plants which treat groundwater may have very little turbidity to remove are unlikely to be affected by weather-related changes in water conditions. As a result, groundwater plants may perform jar tests seldom, if at all, although they can have problems with removing the more difficult small suspended particles typically found in groundwater. Surface water plants, in contrast, tend to treat water with a high turbidity which is susceptible to sudden changes in water quality. Operators at these plants will perform jar tests frequently, especially after rains, to adjust the coagulant dosage and deal with the changing source water turbidity 1.3
Procedure
Note : Prior to the jar test procedure each 500ml sample must be corrected to the required pH using H2SO4 or NaOH .In order to obtain the correct amount of acid or alkali for each sample a separate titration must be carried out for each alum dose-pH combination. Use 100ml water samples for your titration. Determine the pH and turbidity of the raw water. Test for optimum dose (pH constant
i) Fill six 1 liter beakers with 500ml the given kaolin (clay) suspension (SS about 50 mg/l).
ii) Fill a burrete with 50ml alum solution of concentration 2.0 g/L.
iii) Add to the beakers the amount of H2SO4 or NaOH that would yield a final pH of 7.0 (Fill the amount of H2SO4 or NaOH used in Table 2.
iv) Add to the beakers alum solution corresponding to doses of 0 (control), 20, 40, 60, 80 and 100 mg/l.
v) Mix the samples at high speed (80 rpm) for 1 minute.
vi) Reduce mixing speed to 30 rpm and continue mixing for 15 minutes.
vii) Stop the stirrer and let the flocs settle for 20 minutes.
viii) Determine turbidity of supernatant.
ix) Plot turbidity vs. alum dose
RESULTS : EXPERIMENT : Optimum dose (pH constant) Sample volume =
ml
Alum concentration = Beaker
g/liter
Volume of Alum
Alum dose (mg/L)
(ml)
Turbidity (NTU) (from 100ml supernatant)
1
0
0
0
2
5
20
124
3
10
40
76.7 96.6
4
15
60
5
20
80
89.6
6
25
100
140
DISCUSSION :
CONCLUSION :
QUESTIONS
1. Name some other chemicals that may be used as coagulants. 2. In a jar test experiment, why does the turbidity increase again after reaching a minimum value? 3. Briefly discuss the effect of alkalinity on coagulation.
1.1
i. ii. iii.
1.2
Objective
To determine the optimum alum dose pH for suspended solids removal from water using jar test. To determine the optimum concentration of coagulant to be added to the source water.
Coagulation and Flocculation
Suspended solids (particularly colloids) are often removed from water by chemical coagulation and flocculation. Colloidal particles carry a small electrostatic charge that keeps them in suspension. Coagulation is the addition of chemicals to neutralize the effect of colloidal charges and allow aggregation of particles. Following coagulation the suspension is stirred gently to promote particle collision and agglomeration in a process termed flocculation.
The type of source water will have a large impact on how often jar tests are performed. Plants which treat groundwater may have very little turbidity to remove are unlikely to be affected by weather-related changes in water conditions. As a result, groundwater plants may perform jar tests seldom, if at all, although they can have problems with removing the more difficult small suspended particles typically found in groundwater. Surface water plants, in contrast, tend to treat water with a high turbidity which is susceptible to sudden changes in water quality. Operators at these plants will perform jar tests frequently, especially after rains, to adjust the coagulant dosage and deal with the changing source water turbidity 1.3
Procedure
Note : Prior to the jar test procedure each 500ml sample must be corrected to the required pH using H2SO4 or NaOH .In order to obtain the correct amount of acid or alkali for each sample a separate titration must be carried out for each alum dose-pH combination. Use 100ml water samples for your titration. Determine the pH and turbidity of the raw water. Test for optimum dose (pH constant
i) Fill six 1 liter beakers with 500ml the given kaolin (clay) suspension (SS about 50 mg/l).
ii) Fill a burrete with 50ml alum solution of concentration 2.0 g/L.
iii) Add to the beakers the amount of H2SO4 or NaOH that would yield a final pH of 7.0 (Fill the amount of H2SO4 or NaOH used in Table 2.
iv) Add to the beakers alum solution corresponding to doses of 0 (control), 20, 40, 60, 80 and 100 mg/l.
v) Mix the samples at high speed (80 rpm) for 1 minute.
vi) Reduce mixing speed to 30 rpm and continue mixing for 15 minutes.
vii) Stop the stirrer and let the flocs settle for 20 minutes.
viii) Determine turbidity of supernatant.
ix) Plot turbidity vs. alum dose
RESULTS : EXPERIMENT : Optimum dose (pH constant) Sample volume =
ml
Alum concentration = Beaker
g/liter
Volume of Alum
Alum dose (mg/L)
(ml)
Turbidity (NTU) (from 100ml supernatant)
1
0
0
0
2
5
20
124
3
10
40
76.7 96.6
4
15
60
5
20
80
89.6
6
25
100
140
DISCUSSION :
CONCLUSION :
QUESTIONS
1. Name some other chemicals that may be used as coagulants. 2. In a jar test experiment, why does the turbidity increase again after reaching a minimum value? 3. Briefly discuss the effect of alkalinity on coagulation.